Connecting the ear to the brain: Molecular mechanisms of auditory circuit assembly
- PMID: 21232575
- PMCID: PMC3078955
- DOI: 10.1016/j.pneurobio.2011.01.004
Connecting the ear to the brain: Molecular mechanisms of auditory circuit assembly
Abstract
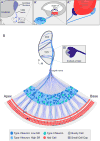
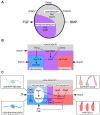
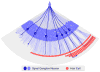
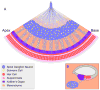
Our sense of hearing depends on precisely organized circuits that allow us to sense, perceive, and respond to complex sounds in our environment, from music and language to simple warning signals. Auditory processing begins in the cochlea of the inner ear, where sounds are detected by sensory hair cells and then transmitted to the central nervous system by spiral ganglion neurons, which faithfully preserve the frequency, intensity, and timing of each stimulus. During the assembly of auditory circuits, spiral ganglion neurons establish precise connections that link hair cells in the cochlea to target neurons in the auditory brainstem, develop specific firing properties, and elaborate unusual synapses both in the periphery and in the CNS. Understanding how spiral ganglion neurons acquire these unique properties is a key goal in auditory neuroscience, as these neurons represent the sole input of auditory information to the brain. In addition, the best currently available treatment for many forms of deafness is the cochlear implant, which compensates for lost hair cell function by directly stimulating the auditory nerve. Historically, studies of the auditory system have lagged behind other sensory systems due to the small size and inaccessibility of the inner ear. With the advent of new molecular genetic tools, this gap is narrowing. Here, we summarize recent insights into the cellular and molecular cues that guide the development of spiral ganglion neurons, from their origin in the proneurosensory domain of the otic vesicle to the formation of specialized synapses that ensure rapid and reliable transmission of sound information from the ear to the brain.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures
References
-
- Abello G, Alsina B. Establishment of a proneural field in the inner ear. Int J Dev Biol. 2007;51:483–493. - PubMed
-
- Abello G, Khatri S, Radosevic M, Scotting PJ, Giraldez F, Alsina B. Independent regulation of Sox3 and Lmx1b by FGF and BMP signaling influences the neurogenic and non-neurogenic domains in the chick otic placode. Dev Biol. 2010;339:166–178. - PubMed
-
- Adam J, Myat A, Le Roux I, Eddison M, Henrique D, Ish-Horowicz D, Lewis J. Cell fate choices and the expression of Notch, Delta and Serrate homologues in the chick inner ear: parallels with Drosophila sense-organ development. Development. 1998;125:4645–4654. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

