Data-driven optimization and evaluation of 2D EPI and 3D PRESTO for BOLD fMRI at 7 Tesla: I. Focal coverage
- PMID: 21232613
- PMCID: PMC3049844
- DOI: 10.1016/j.neuroimage.2010.12.086
Data-driven optimization and evaluation of 2D EPI and 3D PRESTO for BOLD fMRI at 7 Tesla: I. Focal coverage
Abstract
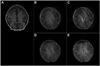
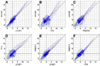
Blood oxygenation level dependent (BOLD) functional magnetic resonance imaging (fMRI) is commonly performed using 2D single-shot echo-planar imaging (EPI). However, single-shot EPI at 7 Tesla (T) often suffers from significant geometric distortions (due to low bandwidth (BW) in the phase-encode (PE) direction) and amplified physiological noise. Recent studies have suggested that 3D multi-shot sequences such as PRESTO may offer comparable BOLD contrast-to-noise ratio with increased volume coverage and decreased geometric distortions. Thus, a four-way group-level comparison was performed between 2D and 3D acquisition sequences at two in-plane resolutions. The quality of fMRI data was evaluated via metrics of prediction and reproducibility using NPAIRS (Non-parametric Prediction, Activation, Influence and Reproducibility re-Sampling). Group activation maps were optimized for each acquisition strategy by selecting the number of principal components that jointly maximized prediction and reproducibility, and showed good agreement in sensitivity and specificity for positive BOLD changes. High-resolution EPI exhibited the highest z-scores of the four acquisition sequences; however, it suffered from the lowest BW in the PE direction (resulting in the worst geometric distortions) and limited spatial coverage, and also caused some subject discomfort through peripheral nerve stimulation (PNS). In comparison, PRESTO also had high z-scores (higher than EPI for a matched in-plane resolution), the highest BW in the PE direction (producing images with superior geometric fidelity), the potential for whole-brain coverage, and no reported PNS. This study provides evidence to support the use of 3D multi-shot acquisition sequences in lieu of single-shot EPI for ultra high field BOLD fMRI at 7T.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures


References
-
- Andersen AH, Gash DM, Avison MJ. Principal component analysis of the dynamic response measured by fMRI: a generalized linear systems framework. Magn. Reson. Imag. 1999;17:795–815. - PubMed
-
- Bowen CV, Menon RS, Gati JS. High field balanced-SSFP fMRI: a BOLD technique with excellent tissue sensitivity and superior large vessel suppression. Proc. 13th Scientific Meeting of the ISMRM; Miami Beach, Florida. 2005. p. 119.
-
- Cohen MS, Weisskoff RM, Rzedzian RR, Kantor HL. Sensory stimulation by time-varying magnetic fields. Magn. Reson. Med. 1990;14:409–414. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

