Immunohistochemical characterization of the intrinsic cardiac neural plexus in whole-mount mouse heart preparations
- PMID: 21232628
- PMCID: PMC3081960
- DOI: 10.1016/j.hrthm.2011.01.013
Immunohistochemical characterization of the intrinsic cardiac neural plexus in whole-mount mouse heart preparations
Abstract
Background: The intrinsic neural plexus of the mouse heart has not been adequately investigated despite the extensive use of this species in experimental cardiology.
Objective: The purpose of this study was to determine the distribution of cholinergic, adrenergic, and sensory neural components in whole-mount mouse heart preparations using double immunohistochemical labeling.
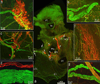
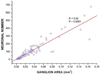
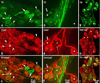
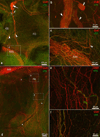
Methods/results: Intrinsic neurons were concentrated within 19 ± 3 ganglia (n = 20 mice) of varying size, scattered on the medial side of the inferior caval (caudal) vein on the right atrium and close to the pulmonary veins on the left atrium. Of a total of 1,082 ± 160 neurons, most somata (83%) were choline acetyltransferase (ChAT) immunoreactive, whereas 4% were tyrosine hydroxylase (TH) immunoreactive; 14% of ganglionic cells were biphenotypic for ChAT and TH. The most intense ChAT staining was observed in axonal varicosities. ChAT was evident in nerve fibers interconnecting intrinsic ganglia. Both ChAT and TH immunoreactivity were abundant within the nerves accessing the heart. However, epicardial TH-immunoreactive nerve fibers were predominant on the dorsal and ventral left atrium, whereas most ChAT-positive axons proceeded on the heart base toward the large intrinsic ganglia and on the epicardium of the root of the right cranial vein. Substance P-positive and calcitonin gene-related peptide-immunoreactive nerve fibers were abundant on the epicardium and within ganglia adjacent to the heart hilum. Small intensely fluorescent cells were grouped into clusters of 3 to 8 and were dispersed within large ganglia or separately on the atrial and ventricular walls.
Conclusion: Although some nerves and neuronal bundles of the mouse epicardial plexus are mixed, most express either adrenergic or cholinergic markers. Therefore, selective stimulation and/or ablation of the functionally distinct intrinsic neural pathways should allow the study of specific effects on cardiac function.
Copyright © 2011 Heart Rhythm Society. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
No Conflicts
Figures

Comment in
-
Physiology of the intrinsic cardiac nervous system.Heart Rhythm. 2011 May;8(5):739. doi: 10.1016/j.hrthm.2011.01.033. Epub 2011 Jan 26. Heart Rhythm. 2011. PMID: 21277999 No abstract available.
Similar articles
-
Electrophysiological effects of nicotinic and electrical stimulation of intrinsic cardiac ganglia in the absence of extrinsic autonomic nerves in the rabbit heart.Heart Rhythm. 2018 Nov;15(11):1698-1707. doi: 10.1016/j.hrthm.2018.05.018. Epub 2018 May 23. Heart Rhythm. 2018. PMID: 29800749 Free PMC article.
-
Innervation of the rabbit cardiac ventricles.J Anat. 2016 Jan;228(1):26-46. doi: 10.1111/joa.12400. Epub 2015 Oct 29. J Anat. 2016. PMID: 26510903 Free PMC article.
-
Morphologic pattern of the intrinsic ganglionated nerve plexus in mouse heart.Heart Rhythm. 2011 Mar;8(3):448-54. doi: 10.1016/j.hrthm.2010.11.019. Epub 2010 Nov 12. Heart Rhythm. 2011. PMID: 21075216 Free PMC article.
-
The heart's 'little brain' controlling cardiac function in the rabbit.Exp Physiol. 2015 Apr 1;100(4):348-53. doi: 10.1113/expphysiol.2014.080168. Epub 2014 Oct 29. Exp Physiol. 2015. PMID: 25833107 Free PMC article. Review.
-
Characterization of the intrinsic cardiac nervous system.Auton Neurosci. 2016 Aug;199:3-16. doi: 10.1016/j.autneu.2016.08.006. Epub 2016 Aug 6. Auton Neurosci. 2016. PMID: 27568996 Review.
Cited by
-
Topographical mapping of catecholaminergic axon innervation in the flat-mounts of the mouse atria: a quantitative analysis.Sci Rep. 2023 Apr 7;13(1):4850. doi: 10.1038/s41598-023-27727-9. Sci Rep. 2023. PMID: 37029119 Free PMC article.
-
Sensory ataxia and cardiac hypertrophy caused by neurovascular oxidative stress in chemogenetic transgenic mouse lines.Nat Commun. 2023 May 29;14(1):3094. doi: 10.1038/s41467-023-38961-0. Nat Commun. 2023. PMID: 37248315 Free PMC article.
-
Electrophysiological effects of nicotinic and electrical stimulation of intrinsic cardiac ganglia in the absence of extrinsic autonomic nerves in the rabbit heart.Heart Rhythm. 2018 Nov;15(11):1698-1707. doi: 10.1016/j.hrthm.2018.05.018. Epub 2018 May 23. Heart Rhythm. 2018. PMID: 29800749 Free PMC article.
-
Electrical properties and synaptic transmission in mouse intracardiac ganglion neurons in situ.Physiol Rep. 2021 Sep;9(18):e15056. doi: 10.14814/phy2.15056. Physiol Rep. 2021. PMID: 34582125 Free PMC article.
-
Anatomical evidence of non-parasympathetic cardiac nitrergic nerve fibres in rat.J Anat. 2021 Jan;238(1):20-35. doi: 10.1111/joa.13291. Epub 2020 Aug 13. J Anat. 2021. PMID: 32790077 Free PMC article.
References
-
- Ardell JL, Butler CK, Smith FM, Hopkins DA, Armour JA. Activity of in vivo atrial and ventricular neurons in chronically decentralized canine hearts. Am J Physiol. 1991;260:H713–H721. - PubMed
-
- Pauza DH, Skripkiene G, Skripka V, Pauziene N, Stropus R. Morphological study of neurons in the nerve plexus on heart base of rats and guinea pigs. J Auton Nerv Syst. 1997;62:1–12. - PubMed
-
- Smith FM. Extrinsic inputs to intrinsic neurons in the porcine heart in vitro. Am J Physiol. 1999;276:R455–R467. - PubMed
-
- Mabe AM, Hoard JL, Duffourc MM, Hoover DB. Localization of cholinergic innervation and neurturin receptors in adult mouse heart and expression of the neurturin gene. Cell Tissue Res. 2006;326:57–67. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

