Adenine nucleotide translocase 1 deficiency results in dilated cardiomyopathy with defects in myocardial mechanics, histopathological alterations, and activation of apoptosis
- PMID: 21232697
- PMCID: PMC4023693
- DOI: 10.1016/j.jcmg.2010.06.018
Adenine nucleotide translocase 1 deficiency results in dilated cardiomyopathy with defects in myocardial mechanics, histopathological alterations, and activation of apoptosis
Abstract
Objectives: the aim of this study was to test the hypothesis that chronic mitochondrial energy deficiency causes dilated cardiomyopathy, we characterized the hearts of age-matched young and old adenine nucleotide translocator (ANT)1 mutant and control mice.
Background: ANTs export mitochondrial adenosine triphosphate into the cytosol and have a role in the regulation of the intrinsic apoptosis pathway. Mitochondrial energy deficiency has been hypothesized, on the basis of indirect evidence, to be a factor in the pathophysiology of dilated cardiomyopathies. Ant1 inactivation should limit adenosine triphosphate for contraction and calcium transport, thereby resulting in early cardiac dysfunction with later dilation and heart failure.
Methods: we conducted a multiyear study of 73 mutant (Ant1-/-) and 57 control (Ant1+/+) mice, between the ages of 2 and 21 months. Hearts were characterized by cardiac anatomy, echocardiographic imaging with velocity vector analysis, histopathology, and apoptosis assays.
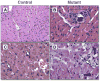
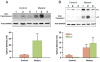
Results: the Ant1-/- mice developed a distinctive concentric dilated cardiomyopathy, characterized by substantial myocardial hypertrophy and ventricular dilation, with cardiac function declining earlier in age as compared to control mice. Left ventricular circumferential, radial, and rotational mechanics were reduced even in the younger mutants with preserved systolic function. Histopathologic analysis demonstrated increased myocyte hypertrophy, fibrosis, and calcification in the mutant mice as compared with control mice. Furthermore, increased cytoplasmic cytochrome c levels and caspase 3 activation were observed in the mutant mice.
Conclusions: our results demonstrate that mitochondrial energy deficiency is sufficient to cause dilated cardiomyopathy, confirming that energy defects are a factor in this disease. Energy deficiency initially leads to early mechanical dysfunction before a decline in left ventricular systolic function. Chronic energy deficiency with age then leads to heart failure. Our results now allow us to use the Ant1-/- mouse model for testing new therapies for ANT1 mutant patients.
2011 American College of Cardiology Foundation. Published by Elsevier Inc. All rights reserved.
Figures




Comment in
-
Human "nuclear" mitochondrial cardiomyopathy a novel mouse model characterizes the disease.JACC Cardiovasc Imaging. 2011 Jan;4(1):11-5. doi: 10.1016/j.jcmg.2010.08.019. JACC Cardiovasc Imaging. 2011. PMID: 21232698 No abstract available.
References
-
- Portman MA. The adenine nucleotide translocator: regulation and function during myocardial development and hypertrophy. Clin Exp Pharmacol. 2002;29:334–8. - PubMed
-
- Pebay-Peyroula E, Dahout-Gonzalez C, Kahn R, et al. Structure of mitochondrial ADP/ATP carrier in complex with carboxyatractyloside. Nature. 2003;426:39–44. - PubMed
-
- Zamora M, Granell M, Mampel T, Vinas O. Adenine nucleotide translocase 3 (ANT3) overexpression induces apoptosis in cultured cells. FEBS Lett. 2004;563:155–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

