Inflammatory signals regulate hematopoietic stem cells
- PMID: 21233016
- PMCID: PMC3042730
- DOI: 10.1016/j.it.2010.12.003
Inflammatory signals regulate hematopoietic stem cells
Abstract
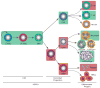
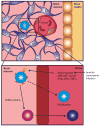
Hematopoietic stem cells (HSCs) are the progenitors of all blood and immune cells, yet their role in immunity is not well understood. Most studies have focused on the ability of committed lymphoid and myeloid precursors to replenish immune cells during infection. Recent studies, however, have indicated that HSCs also proliferate in response to systemic infection and replenish effector immune cells. Inflammatory signaling molecules including interferons, tumor necrosis factor-α and Toll-like receptors are essential to the HSC response. Observing the biology of HSCs through the lens of infection and inflammation has led to the discovery of an array of immune-mediators that serve crucial roles in HSC regulation and function.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures

References
-
- Harrison DE, Lerner CP. Most primitive hematopoietic stem cells are stimulated to cycle rapidly after treatment with 5-fluorouracil. Blood. 1991;78(5):1237–1240. - PubMed
-
- Cheshier SH, et al. The effect of bleeding on hematopoietic stem cell cycling and self-renewal. Stem Cells Dev. 2007;16(5):707–717. - PubMed
-
- Sigvardsson M. New light on the biology and developmental potential of haematopoietic stem cells and progenitor cells. Journal of Internal Medicine. 2009;266(4):311–324. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

