A therapeutic dendritic cell-based vaccine for HIV-1 infection
- PMID: 21233310
- PMCID: PMC3071229
- DOI: 10.1093/infdis/jiq077
A therapeutic dendritic cell-based vaccine for HIV-1 infection
Abstract
A double-blinded, controlled study of vaccination of untreated patients with chronic human immunodeficiency virus type 1 (HIV-1) infection with 3 doses of autologous monocyte-derived dendritic cells (MD-DCs) pulsed with heat inactivated autologous HIV-1 was performed. Therapeutic vaccinations were feasible, safe, and well tolerated. At week 24 after first vaccination (primary end point), a modest significant decrease in plasma viral load was observed in vaccine recipients, compared with control subjects (P = .03). In addition, the change in plasma viral load after vaccination tended to be inversely associated with the increase in HIV-specific T cell responses in vaccinated patients but tended to be directly correlated with HIV-specific T cell responses in control subjects.
Trial registration: ClinicalTrials.gov NCT00402142.
Figures

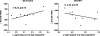
References
-
- Kundu SK, Engleman E, Benike C, et al. A pilot clinical trial of HIV antigen-pulsed allogeneic and autologous dendritic cell therapy in HIV-infected patients. AIDS Res Hum Retroviruses. 1998;14:551–60. - PubMed
-
- Lu W, Arraes L, Ferreira W, Andrieu JM. Therapeutic dendritic-cell vaccine for chronic HIV-1 infection. Nat Med. 2004;10:1359–65. - PubMed
-
- García F, Lejeune M, Climent N, et al. Therapeutic immunization with dendritic cells loaded with inactivated autologous HIV-1 in chronic HIV-1 infected patients. J Infect Dis. 2005;195:1680–5. - PubMed
-
- Ide F, Nakamura T, Tomizawa M, et al. Peptide-loaded dendritic-cell vaccination followed by treatment interruption for chronic HIV-1 infection: a phase 1 trial. J Med Virol. 2006;78:711–8. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

