CD8+ T regulatory cells express the Ly49 Class I MHC receptor and are defective in autoimmune prone B6-Yaa mice
- PMID: 21233417
- PMCID: PMC3033298
- DOI: 10.1073/pnas.1018974108
CD8+ T regulatory cells express the Ly49 Class I MHC receptor and are defective in autoimmune prone B6-Yaa mice
Abstract
The immune system includes a subpopulation of CD8(+) T cells equipped to inhibit the expansion of follicular T helper (T(FH)) cells, resulting in suppression of autoantibody production and associated lupus-like disease. These CD8(+) T regulatory (Treg) cells recognize Qa-1/peptide complexes on target T(FH) cells and depend on the IL-15 cytokine for development and function. Here we show that these CD8(+) Treg cells express a triad of surface receptors--CD44, CD122, and the class I MHC receptor Ly49--and account for <5% of CD8(+) T cells. Moreover, the development of systemic lupus erythematosus-like disease in B6-Yaa mutant mice is associated with a pronounced defect in CD8(+) Treg cell activity, suggesting that this regulatory subset may represent an effective therapeutic approach to systemic lupus erythematosus-like autoimmune disease.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
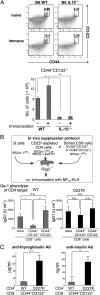


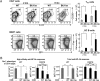
References
-
- Bouneaud C, Kourilsky P, Bousso P. Impact of negative selection on the T cell repertoire reactive to a self-peptide: A large fraction of T cell clones escapes clonal deletion. Immunity. 2000;13:829–840. - PubMed
-
- Goldrath AW, Bevan MJ. Selecting and maintaining a diverse T-cell repertoire. Nature. 1999;402:255–262. - PubMed
-
- Slifka MK, et al. Preferential escape of subdominant CD8+ T cells during negative selection results in an altered antiviral T cell hierarchy. J Immunol. 2003;170:1231–1239. - PubMed
-
- Kearney ER, Pape KA, Loh DY, Jenkins MK. Visualization of peptide-specific T cell immunity and peripheral tolerance induction in vivo. Immunity. 1994;1:327–339. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

