Targeted delivery of a cisplatin prodrug for safer and more effective prostate cancer therapy in vivo
- PMID: 21233423
- PMCID: PMC3033286
- DOI: 10.1073/pnas.1011379108
Targeted delivery of a cisplatin prodrug for safer and more effective prostate cancer therapy in vivo
Abstract
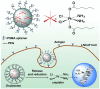
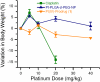
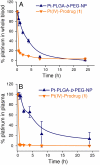
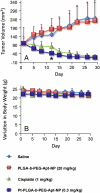
Targeted delivery and controlled release of inactive platinum (Pt) prodrugs may offer a new approach to improve the efficacy and tolerability of the Pt family of drugs, which are used to treat 50% of all cancers today. Using prostate cancer (PCa) as a model disease, we previously described the engineering of aptamer (Apt)-targeted poly(D,L-lactic-co-glycolic acid)-b-poly(ethylene glycol) (PLGA-b-PEG) nanoparticles (NPs) encapsulating a Pt(IV) prodrug c,t,c[Pt(NH(3))(2)-(O(2)CCH(2)CH(2)CH(2)CH(2)CH(3))(2)Cl(2)] (1) (Pt-PLGA-b-PEG-Apt-NP), which target the extracellular domain of the prostate specific membrane antigen (PSMA), for enhanced in vitro cytotoxicity. Here we demonstrate enhanced in vivo pharmacokinetics (PK), biodistribution, tolerability, and efficacy of Pt-PLGA-b-PEG-Apt-NP (150 ± 15 nm encapsulating ∼5% wt/wt Pt(IV) prodrug) when compared to cisplatin administered in its conventional form in normal Sprague Dawley rats, Swiss Albino mice, and the PSMA-expressing LNCaP subcutaneous xenograft mouse model of PCa, respectively. The 10-d maximum tolerated dose following a single i.v. injection of Pt-PLGA-b-PEG-NP in rats and mice was determined at 40 mg/kg and 5 mg/kg, respectively. PK studies with Pt-PLGA-b-PEG-NP revealed prolonged Pt persistence in systemic blood circulation and decreased accumulation of Pt in the kidneys, a major target site of cisplatin toxicity. Pt-PLGA-b-PEG-Apt-NPs further displayed the significant dose-sparing characteristics of the drug, with equivalent antitumor efficacy in LNCaP xenografts at 1/3 the dose of cisplatin administered in its conventional form (0.3 mg/kg vs. 1 mg/kg). When considering the simultaneous improvement in tolerability and efficacy, the Pt-PLGA-b-PEG-Apt NP provides a remarkable improvement in the drug therapeutic index.
Conflict of interest statement
Conflict of interest statement: In compliance with the Brigham and Women’s Hospital and Harvard Medical School institutional guidelines, O.C.F. discloses his financial interest in BIND Biosciences and Selecta Biosciences, two biotechnology companies developing nanoparticle technologies for medical applications. BIND and Selecta did not support the aforementioned research, and currently these companies have no rights to any technology or intellectual property developed as part of this research.
Figures
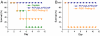

References
-
- Chen Y, Tang Y, Wang MT, Zeng S, Nie D. Human pregnane X receptor and resistance to chemotherapy in prostate cancer. Cancer Res. 2007;67:10361–10367. - PubMed
-
- Jamieson ER, Lippard SJ. Structure, recognition, and processing of cisplatin-DNA adducts. Chem Rev. 1999;99:2467–2498. - PubMed
-
- Rosenberg B, VanCamp L, Trosko JE, Mansour VH. Platinum compounds: A new class of potent antitumour agents. Nature. 1969;222:385–386. - PubMed
-
- Galanski M, Jakupec MA, Keppler BK. Update of the preclinical situation of anticancer platinum complexes: Novel design strategies and innovative analytical approaches. Curr Med Chem. 2005;12:2075–2094. - PubMed
-
- Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov. 2005;4:307–320. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

