Influence of body weight on achieving indinavir concentrations within its therapeutic window in HIV-infected Thai patients receiving indinavir boosted with ritonavir
- PMID: 21233689
- PMCID: PMC3058116
- DOI: 10.1097/FTD.0b013e3182057f6f
Influence of body weight on achieving indinavir concentrations within its therapeutic window in HIV-infected Thai patients receiving indinavir boosted with ritonavir
Abstract
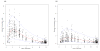
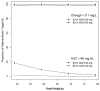
Indinavir boosted with ritonavir (IDV/r) dosing with 400/100 mg, twice daily, is preferred in Thai adults, but this dose can lead to concentrations close to the boundaries of its therapeutic window. The objectives of this analysis were to validate a population pharmacokinetic model to describe IDV/r concentrations in HIV-infected Thai patients and to investigate the impact of patient characteristics on achieving adequate IDV concentrations. IDV/r concentration data from 513 plasma samples were available. Population means and variances of pharmacokinetic parameters were estimated using a nonlinear mixed effects regression model (NONMEM Version VI). Monte Carlo simulations were performed to estimate the probability of achieving IDV concentrations within its therapeutic window. IDV/r pharmacokinetics were best described by a one-compartment model coupled with a single transit compartment absorption model. Body weight influenced indinavir apparent oral clearance and volume of distribution and allometric scaling significantly reduced the interindividual variability. Final population estimates (interindividual variability in percentage) of indinavir apparent oral clearance and volume of distribution were 21.3 L/h/70 kg (30%) and 90.7 L/70 kg (22%), respectively. Based on model simulations, the probability of achieving an IDV trough concentration greater than 0.1 mg/L was greater than 99% for 600/100 mg and greater than 98% for 400/100 mg, twice daily, in patients weighing 40 to 80 kg. However, the probability of achieving IDV concentrations associated with an increased risk of drug toxicity (greater than 10.0 mg/L) increased from 1% to 10% with 600/100 mg compared with less than 1% with 400/100 mg when body weight decreased from 80 to 40 kg. The validated model developed predicts that 400/100 mg of IDV/r, twice daily, provides indinavir concentrations within the recommended therapeutic window for the majority of patients. The risk of toxic drug concentrations increases rapidly with IDV/r dose of 600/100 mg for patients less than 50 kg and therapeutic drug monitoring of IDV concentrations would help to reduce the risk of IDV-induced nephrotoxicity.
Figures
References
-
- World Health Organization. Antiretroviral Therapy for HIV Infection in Infants and Children: Towards Universal Access. Recommendations for a public health approach. 2010. http://www.who.int/hiv/pub/paediatric/infants2010/en/index.html. Revision. - PubMed
-
- Hammer SM, Squires KE, Hughes MD, et al. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. AIDS Clinical Trials Group 320 Study Team. N Engl J Med. 1997;337:725–733. - PubMed
-
- Gulick RM, Mellors JW, Havlir D, et al. Treatment with indinavir, zidovudine, and lamivudine in adults with human immunodeficiency virus infection and prior antiretroviral therapy. N Engl J Med. 1997;337:734–739. - PubMed
-
- Arnaiz JA, Mallolas J, Podzamczer D, et al. Continued indinavir versus switching to indinavir/ritonavir in HIV-infected patients with suppressed viral load. Aids. 2003;17:831–840. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

