Treatment of disseminated mycobacterial infection with high-dose IFN-γ in a patient with IL-12Rβ1 deficiency
- PMID: 21234109
- PMCID: PMC3014684
- DOI: 10.1155/2011/691956
Treatment of disseminated mycobacterial infection with high-dose IFN-γ in a patient with IL-12Rβ1 deficiency
Abstract
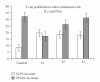
IFN-γ has been used in the treatment of IL-12Rβ1 deficiency patients with disseminated BCG infection (BCGosis), but the optimal dose to reach efficacy is not clear. We used IFN-γ in the treatment of a 2.7-year-old patient with IL-12Rβ1 deficiency and refractory BCG-osis. IFNγ was started at a dose of 50 μg/m² 3 times per week. The dose was upgraded to 100 mcg/m² after 3 months, then to 200 mcg/m² 6 months afterwards. Serum mycobactericidal activity and lymphocytes number and function were evaluated throughout the study. There was no clinical response to IFN-γ with 50 or 100 μg/m² doses. However, there was some response to the 200 μg/m² dose with no additional adverse effects. The serum mycobactericidal activity was not significantly different during the whole treatment period. Lymphocytes proliferation in response to PHA was significantly higher after 3 months of using the highest dose as compared to the lowest dose. The tuberculin skin test reaction remained persistently negative. We conclude that in a patient with IL-12Rβ1 deficiency, IFN-γ at a dose of 200 μg/m², but not at lower dosages, was found to have a noticeable clinical effect with no additional adverse effects.
Figures
References
-
- Casanova JL, Abel L. Genetic dissection of immunity to mycobacteria: the human model. Annual Review of Immunology. 2002;20:581–620. - PubMed
-
- Filipe-Santos O, Bustamante J, Chapgier A, et al. Inborn errors of IL-12/23- and IFN-γ-mediated immunity: molecular, cellular, and clinical features. Seminars in Immunology. 2006;18(6):347–361. - PubMed
-
- Al-Muhsen S, Casanova JL. The genetic heterogeneity of mendelian susceptibility to mycobacterial diseases. Journal of Allergy and Clinical Immunology. 2008;122(6):1043–1051. - PubMed
-
- Altare F, Durandy A, Lammas D, et al. Impairment of mycobacterial immunity in human interleukin-12 receptor deficiency. Science. 1998;280(5368):1432–1435. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

