Xenotransplantation of embryonic pig kidney or pancreas to replace the function of mature organs
- PMID: 21234246
- PMCID: PMC3018651
- DOI: 10.1155/2011/501749
Xenotransplantation of embryonic pig kidney or pancreas to replace the function of mature organs
Abstract
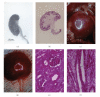
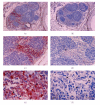
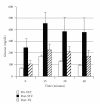
Lack of donor availability limits the number of human donor organs. The need for host immunosuppression complicates transplantation procedures. Ultrastructurally precise kidneys differentiate in situ following xenotransplantation in mesentery of embryonic pig renal primordia. The developing organ attracts its blood supply from the host, obviating humoral rejection. Engraftment of pig renal primordia transplanted directly into rats requires host immune suppression. However, insulin-producing cells originating from embryonic pig pancreas obtained very early following initiation of organogenesis [embryonic day 28 (E28)] engraft long term in nonimmune-suppressed diabetic rats or rhesus macaques. Engraftment of morphologically similar cells originating from adult porcine islets of Langerhans (islets) occurs in rats previously transplanted with E28 pig pancreatic primordia. Here, we review recent findings germane to xenotransplantation of pig renal or pancreatic primordia as a novel organ replacement strategy.
Figures


References
-
- Brands K, Colvin E, Williams LJ, Wang R, Lock RB, Tuch BE. Reduced immunogenicity of first-trimester human fetal pancreas. Diabetes. 2008;57(3):627–634. - PubMed
-
- Dekel B, Amariglio N, Kaminski N, et al. Engraftment and differentiation of human metanephroi into functional mature nephrons after transplantation into mice is accompanied by a profile of gene expression similar to normal human kidney development. Journal of the American Society of Nephrology. 2002;13(4):977–990. - PubMed
-
- Dekel B, Burakova T, Arditti FD, et al. Human and porcine early kidney precursors as a new source for transplantation. Nature Medicine. 2003;9(1):53–60. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

