Transition metal-catalyzed decarboxylative allylation and benzylation reactions
- PMID: 21235271
- PMCID: PMC3116714
- DOI: 10.1021/cr1002744
Transition metal-catalyzed decarboxylative allylation and benzylation reactions
Abstract
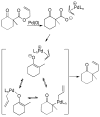
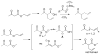
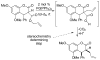
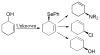
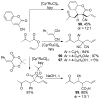
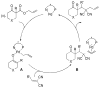
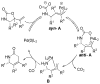
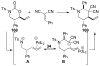
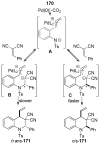
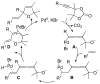
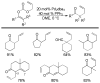
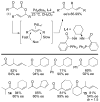
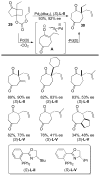
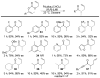
A review. Transition metal catalyzed decarboxylative allylations, benzylations, and interceptive allylations are reviewed.
Figures


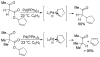
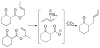
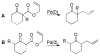
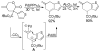
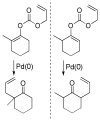
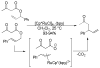
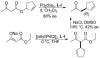


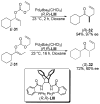






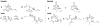


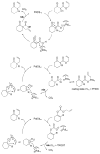


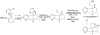

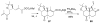




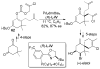



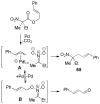
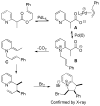




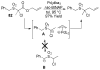
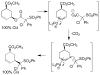

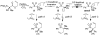
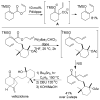


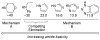


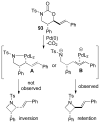
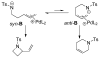



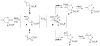
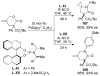



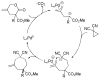





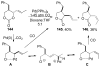

















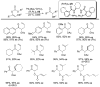



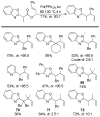
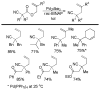


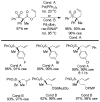
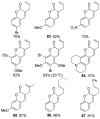
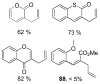
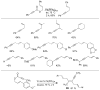
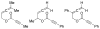

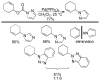


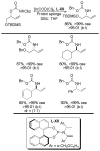
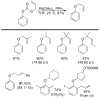
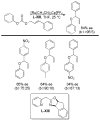






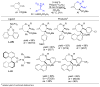
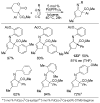




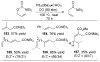
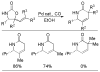



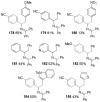
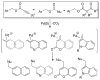


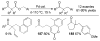

References
-
- Negishi E-i, Wang G, Rao H, Xu Z. J Org Chem. 2010;75:3151. - PMC - PubMed
- Terao J, Kambe N. Acc Chem Res. 2008;41:1545. - PubMed
- Martin R, Buchwald SL. Acc Chem Res. 2008;41:1461. - PMC - PubMed
- Marion N, Nolan SP. Acc Chem Res. 2008;41:1440. - PubMed
- Denmark SE, Regens CS. Acc Chem Res. 2008;41:1486. - PMC - PubMed
- Kantchev EAB, O’Brien CJ, Organ MG. Angew Chem Int Ed. 2007;46:2768. - PubMed
- Phan NTS, Sluys MVD, Jones CW. Adv Synth Catal. 2006;348:609.
- Corbet JP, Mignani G. Chem Rev. 2006;106:2651. - PubMed
- Trost BM. J Org Chem. 2004;69:5813. - PubMed
- Echavarren AM, Cárdenas DJ. In: Metal-Catalyzed Cross-Coupling Reactions. 2. Meijere A, Diederich F, editors. Wiley; 2004.
- Trost BM, Crawley ML. Chem Rev. 2003;103:2921. - PubMed
- Hassan J, Sévignon M, Gozzi C, Schulz E, Lemaire M. Chem Rev. 2002;102:1359. - PubMed
-
- Lyons TW, Sanford MS. Chem Rev. 2010;110:1147. - PMC - PubMed
- Mkhalid IAI, Barnard JH, Marder TB, Murphy JM, Hartwig JF. Chem Rev. 2010;110:890. - PubMed
- Colby DA, Bergman RG, Ellman JA. Chem Rev. 2010;110:624. - PMC - PubMed
- Chen X, Engle KM, Wang DH, Yu JQ. Angew Chem Int Ed. 2009;48:5094. - PMC - PubMed
- Arndtsen BA, Bergman RG, Mobley TA, Peterson TH. Acc Chem Res. 1995;28:154.
-
- Austeri M, Buron F, Constant S, Lacour J, Linder D, Muller J, Tortoioli S. Pure Appl Chem. 2008;80:967.
- Mohr JT, Stoltz BM. Chem Asian J. 2007;2:1476. - PMC - PubMed
- Mohr JT, Ebner DC, Stoltz BM. Org Biomol Chem. 2007;5:3571. - PMC - PubMed
- You SL, Dai LX. Angew Chem Int Ed. 2006;45:5246. - PubMed
- Tunge JA, Burger EC. Eur J Org Chem. 2005:1715.
- Tsuji J. Proc Jpn Acad, B. 2004;80:349.
-
- Tsuji J, Takahashi H, Morikawa M. Tetrahedron Lett. 1965;6:4387.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

