Preserving protein profiles in tissue samples: differing outcomes with and without heat stabilization
- PMID: 21236297
- PMCID: PMC3299412
- DOI: 10.1016/j.jneumeth.2011.01.004
Preserving protein profiles in tissue samples: differing outcomes with and without heat stabilization
Abstract
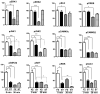
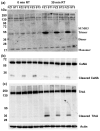
Post translational modification (PTM) and proteolytic processing of proteins contributes to regulation of their stability, intracellular localization and interactions with other proteins, and to direct enhancement or repression of their activity. Both PTM and proteolysis are dynamic; levels and rates change in response to changes in the cellular environment. Tissue excision, post mortem interval and subsequent methods of tissue processing for protein analysis unavoidably alter the cellular environment and therefore features of protein profiles. To aid in understanding the time frame and protein specificity of these changes and the biological and technical contributions to them, we have compared features of protein profiles in mouse hippocampus and cortex following three methods of tissue handling: immediate lysate preparation, and rapid heating to 95°C and standard snap freezing in liquid nitrogen, prior to lysate preparation. In spite of the very short time frames involved, we observe protein-specific differences in levels of phosphorylation, general differences in patterns of sumoylation, and specific differences in levels of proteolytic cleavage of calcineurin and the neurotrophin receptor, TRKA. These differences vary with brain region and with post excision time to processing, and highlight the challenges inherent in accurately profiling the in vivo proteome.
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures




References
-
- Deribe YL, Pawson T, Dikic I. Post-translational modifications in signal integration. Nat Struct Mol Biol. 2010;17:666–672. - PubMed
-
- Díaz-Rodríguez E, Cabrera N, Esparís-Ogando A, Montero JC, Pandiella A. Cleavage of the TrkA neurotrophin receptor by multiple metalloproteases generates signalling-competent truncated forms. Eur J Neurosci. 1999;11:1421–1430. - PubMed
-
- Ferrer I, Santpere G, Arzberger T, Bell J, Blanco R, Boluda S, Budka H, Carmona M, Giaccone G, Krebs B, Limido L, Parchi P, Puig B, Strammiello R, Strobel T, Kretzschmar H. Brain protein preservation largely depends on the postmortem staorage temperature: Implications for study of proteins in human neurologic diseases and management of brain banks: A brainNet Europe study. J Neuropathol Exp Neurol. 2007;66:35–46. - PubMed
-
- Lee JE, Kim H, Jang H, Cho EJ, Youn HD. Hydrogen peroxide triggers the proteolytic cleavage and the inactivation of calcineurin. J Neurochem. 2007;100:1703–1712. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

