Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial
- PMID: 21236730
- PMCID: PMC3033533
- DOI: 10.1016/S1470-2045(10)70290-4
Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial
Abstract
Background: Xerostomia is the most common late side-effect of radiotherapy to the head and neck. Compared with conventional radiotherapy, intensity-modulated radiotherapy (IMRT) can reduce irradiation of the parotid glands. We assessed the hypothesis that parotid-sparing IMRT reduces the incidence of severe xerostomia.
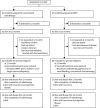
Methods: We undertook a randomised controlled trial between Jan 21, 2003, and Dec 7, 2007, that compared conventional radiotherapy (control) with parotid-sparing IMRT. We randomly assigned patients with histologically confirmed pharyngeal squamous-cell carcinoma (T1-4, N0-3, M0) at six UK radiotherapy centres between the two radiotherapy techniques (1:1 ratio). A dose of 60 or 65 Gy was prescribed in 30 daily fractions given Monday to Friday. Treatment was not masked. Randomisation was by computer-generated permuted blocks and was stratified by centre and tumour site. Our primary endpoint was the proportion of patients with grade 2 or worse xerostomia at 12 months, as assessed by the Late Effects of Normal Tissue (LENT SOMA) scale. Analyses were done on an intention-to-treat basis, with all patients who had assessments included. Long-term follow-up of patients is ongoing. This study is registered with the International Standard Randomised Controlled Trial register, number ISRCTN48243537.
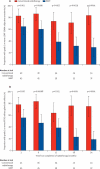
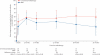
Findings: 47 patients were assigned to each treatment arm. Median follow-up was 44·0 months (IQR 30·0-59·7). Six patients from each group died before 12 months and seven patients from the conventional radiotherapy and two from the IMRT group were not assessed at 12 months. At 12 months xerostomia side-effects were reported in 73 of 82 alive patients; grade 2 or worse xerostomia at 12 months was significantly lower in the IMRT group than in the conventional radiotherapy group (25 [74%; 95% CI 56-87] of 34 patients given conventional radiotherapy vs 15 [38%; 23-55] of 39 given IMRT, p=0·0027). The only recorded acute adverse event of grade 2 or worse that differed significantly between the treatment groups was fatigue, which was more prevalent in the IMRT group (18 [41%; 99% CI 23-61] of 44 patients given conventional radiotherapy vs 35 [74%; 55-89] of 47 given IMRT, p=0·0015). At 24 months, grade 2 or worse xerostomia was significantly less common with IMRT than with conventional radiotherapy (20 [83%; 95% CI 63-95] of 24 patients given conventional radiotherapy vs nine [29%; 14-48] of 31 given IMRT; p<0·0001). At 12 and 24 months, significant benefits were seen in recovery of saliva secretion with IMRT compared with conventional radiotherapy, as were clinically significant improvements in dry-mouth-specific and global quality of life scores. At 24 months, no significant differences were seen between randomised groups in non-xerostomia late toxicities, locoregional control, or overall survival.
Interpretation: Sparing the parotid glands with IMRT significantly reduces the incidence of xerostomia and leads to recovery of saliva secretion and improvements in associated quality of life, and thus strongly supports a role for IMRT in squamous-cell carcinoma of the head and neck.
Funding: Cancer Research UK (CRUK/03/005).
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures

Comment in
-
Reducing xerostomia through advanced technology.Lancet Oncol. 2011 Feb;12(2):110-1. doi: 10.1016/S1470-2045(11)70009-2. Lancet Oncol. 2011. PMID: 21277541 No abstract available.
-
Radiotherapy: IMRT reduces incidence of xerostomia in patients with head and neck cancer.Nat Rev Clin Oncol. 2011 Mar 15;8(4):194. doi: 10.1038/nrclinonc.2011.23. Nat Rev Clin Oncol. 2011. PMID: 21451495 No abstract available.
-
Intensity-modulated radiotherapy in head and neck cancer: how safe is safe?Hematol Oncol Stem Cell Ther. 2011;4(4):192. doi: 10.5144/1658-3876.2011.192. Hematol Oncol Stem Cell Ther. 2011. PMID: 22198191 No abstract available.
-
Oncology scan--demonstrating technology and measuring outcomes in head and neck cancer.Int J Radiat Oncol Biol Phys. 2014 Mar 15;88(4):759-60. doi: 10.1016/j.ijrobp.2013.11.014. Int J Radiat Oncol Biol Phys. 2014. PMID: 24606844 No abstract available.
References
-
- Bhide SA, Nutting CM. Advances in radiotherapy for head and neck cancer. Oral Oncol. 2010;46:439–441. - PubMed
-
- Pignon JP, le Maître A, Maillard E, Bourhis J, MACH-NC Collaborative Group Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92:4–14. - PubMed
-
- Jensen AB, Hansen O, Jorgensen K, Bastholt L. Influence of late side-effects upon daily life after radiotherapy for laryngeal and pharyngeal cancer. Acta Oncol. 1994;33:487–491. - PubMed
-
- Wijers OB, Levendag PC, Braaksma MM, Boonzaaijer M, Visch LL, Schmitz PI. Patients with head and neck cancer cured by radiation therapy: a survey of the dry mouth syndrome in long-term survivors. Head Neck. 2002;24:737–747. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

