The intracellular redox stress caused by hexavalent chromium is selective for proteins that have key roles in cell survival and thiol redox control
- PMID: 21237240
- PMCID: PMC3039098
- DOI: 10.1016/j.tox.2011.01.001
The intracellular redox stress caused by hexavalent chromium is selective for proteins that have key roles in cell survival and thiol redox control
Abstract
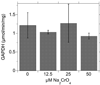
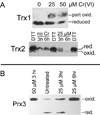
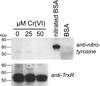
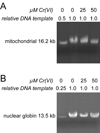
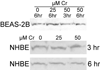
Hexavalent chromium [Cr(VI)] compounds (e.g. chromates) are strong oxidants that readily enter cells where they are reduced to reactive Cr intermediates that can directly oxidize some cell components and can promote the generation of reactive oxygen and nitrogen species. Inhalation is a major route of exposure which directly exposes the bronchial epithelium. Previous studies with non-cancerous human bronchial epithelial cells (BEAS-2B) demonstrated that Cr(VI) treatment results in the irreversible inhibition of thioredoxin reductase (TrxR) and the oxidation of thioredoxins (Trx) and peroxiredoxins (Prx). The mitochondrial Trx/Prx system is somewhat more sensitive to Cr(VI) than the cytosolic Trx/Prx system, and other redox-sensitive mitochondrial functions are subsequently affected including electron transport complexes I and II. Studies reported here show that Cr(VI) does not cause indiscriminant thiol oxidation, and that the Trx/Prx system is among the most sensitive of cellular protein thiols. Trx/Prx oxidation is not unique to BEAS-2B cells, as it was also observed in primary human bronchial epithelial cells. Increasing the intracellular levels of ascorbate, an endogenous Cr(VI) reductant, did not alter the effects on TrxR, Trx, or Prx. The peroxynitrite scavenger MnTBAP did not protect TrxR, Trx, Prx, or the electron transport chain from the effects of Cr(VI), implying that peroxynitrite is not required for these effects. Nitration of tyrosine residues of TrxR was not observed following Cr(VI) treatment, further ruling out peroxynitrite as a significant contributor to the irreversible inhibition of TrxR. Cr(VI) treatments that disrupt the TrxR/Trx/Prx system did not cause detectable mitochondrial DNA damage. Overall, the redox stress that results from Cr(VI) exposure shows selectivity for key proteins which are known to be important for redox signaling, antioxidant defense, and cell survival.
Copyright © 2011 Elsevier Ireland Ltd. All rights reserved.
Figures




References
-
- Arnér ES. Focus on mammalian thioredoxin reductases--important selenoproteins with versatile functions. Biochim. Biophys. Acta. 2009;1790:495–526. - PubMed
-
- Arnér ESJ, Holmgren A. Physiological functions of thioredoxin and thioredoxin reductase. Eur. J. Biochem. 2000;267:6102–6109. - PubMed
-
- Baruthio F. Toxic effects of chromium and its compounds. Biol. Trace Element Res. 1992;32:145–153. - PubMed
-
- Batinic-Haberle I, Cuzzocrea S, Reboucas JS, Ferrer-Sueta G, Mazzon E, Di Paola R, Radi R, Spasojevic I, Benov L, Salvemini D. Pure MnTBAP selectively scavenges peroxynitrite over superoxide: comparison of pure and commercial MnTBAP samples to MnTE-2-PyP in two models of oxidative stress injury, an SOD-specific Escherichia coli model and carrageenan-induced pleurisy. Free Radic. Biol. Med. 2009;46:192–201. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

