Efficacy of RTS,S/AS01E malaria vaccine and exploratory analysis on anti-circumsporozoite antibody titres and protection in children aged 5-17 months in Kenya and Tanzania: a randomised controlled trial
- PMID: 21237715
- PMCID: PMC3341451
- DOI: 10.1016/S1473-3099(10)70262-0
Efficacy of RTS,S/AS01E malaria vaccine and exploratory analysis on anti-circumsporozoite antibody titres and protection in children aged 5-17 months in Kenya and Tanzania: a randomised controlled trial
Erratum in
- Lancet Infect Dis. 2011 Mar;11(3):159
Abstract
Background: RTS,S/AS01E is the lead candidate malaria vaccine. We recently showed efficacy against clinical falciparum malaria in 5-17 month old children, during an average of 8 months follow-up. We aimed to assess the efficacy of RTS,S/AS01E during 15 months of follow-up.
Methods: Between March, 2007, and October, 2008, we enrolled healthy children aged 5-17 months in Kilifi, Kenya, and Korogwe, Tanzania. Computer-generated block randomisation was used to randomly assign participants (1:1) to receive three doses (at month 0, 1, and 2) of either RTS,S/AS01E or human diploid-cell rabies vaccine. The primary endpoint was time to first clinical malaria episode, defined as the presence of fever (temperature ≥37·5°C) and a Plasmodium falciparum density of 2500/μL or more. Follow-up was 12 months for children from Korogwe and 15 months for children from Kilifi. Primary analysis was per protocol. In a post-hoc modelling analysis we characterised the associations between anti-circumsporozoite antibodies and protection against clinical malaria episodes. This study is registered with ClinicalTrials.gov, number NCT00380393.
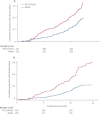
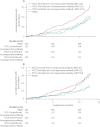
Findings: 894 children were assigned, 447 in each treatment group. In the per-protocol analysis, 82 of 415 children in the RTS,S/AS01E group and 125 of 420 in the rabies vaccine group had first or only clinical malaria episode by 12 months, vaccine efficacy 39·2% (95% CI 19·5-54·1, p=0·0005). At 15 months follow-up, 58 of 209 children in the RTS,S/AS01E group and 85 of 206 in the rabies vaccine group had first or only clinical malaria episode, vaccine efficacy 45·8% (24·1-61·3, p=0·0004). At 12 months after the third dose, anti-circumsporozoite antibody titre data were available for 390 children in the RTS,S/AS01E group and 391 in the rabies group. A mean of 15 months (range 12-18 months) data were available for 172 children in the RTS,S/AS01E group and 155 in the rabies group. These titres at 1 month after the third dose were not associated with protection, but titres at 6·5 months were. The level of protection increased abruptly over a narrow range of antibody concentrations. The most common adverse events were pneumonia, febrile convulsion, gastroenteritis, and P falciparum malaria.
Interpretation: RTS,S/AS01E confers sustained efficacy for at least 15 months and shows promise as a potential public health intervention against childhood malaria in malaria endemic countries.
Funding: PATH Malaria Vaccine Initiative (MVI), GlaxoSmithKline.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures



Comment in
-
Immunological correlates of protection for the RTS,S candidate malaria vaccine.Lancet Infect Dis. 2011 Feb;11(2):75-6. doi: 10.1016/S1473-3099(11)70001-9. Epub 2011 Jan 13. Lancet Infect Dis. 2011. PMID: 21237716 No abstract available.
References
-
- Bryce J, Boschi-Pinto C, Shibuya K, Black RE, WHO Child Health Epidemiology Reference Group WHO estimates of the causes of death in children. Lancet. 2005;365:1147–1152. - PubMed
-
- WHO World malaria report. 2010. http://www.who.int/malaria/publications/atoz/9789241564106/en/index.html (accessed Dec 16, 2010).
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

