Regulation of FOXO protein stability via ubiquitination and proteasome degradation
- PMID: 21238503
- PMCID: PMC3110514
- DOI: 10.1016/j.bbamcr.2011.01.007
Regulation of FOXO protein stability via ubiquitination and proteasome degradation
Abstract
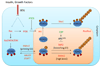
Forkhead box O-class (FOXO) proteins are evolutionally conserved transcription factors. They belong to a family of proteins consisting of FOXO1, FOXO3a, FOXO4 and FOXO6 in humans. Increasing evidence suggests that FOXO proteins function as tumor suppressors by transcriptionally regulating expression of genes involved in cell cycle arrest, apoptosis, DNA repair and oxidative stress resistance. Activation of various protein kinases, including Akt, IκB kinase (IKK) and ERK, leads to phosphorylation of FOXO proteins and their ubiquitination mediated by E3 ligases such as SKP2 and MDM2 in human primary tumors and cancer cell lines. As a result, the tumor suppressor functions of FOXO proteins are either diminished or abrogated due to their ubiquitination-proteasome degradation, thereby favoring cell transformation, proliferation and survival. Thus, ubiquitination and proteasome degradation of FOXO proteins play an important role in tumorigenesis and represent a viable target for cancer treatment. This article is part of a Special Issue entitled: PI3K-AKT-FoxO axis in cancer and aging.
2011 Elsevier B.V. All rights reserved.
Figures

References
-
- Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, Bigner SH, Giovanella BC, Ittmann M, Tycko B, Hibshoosh H, Wigler MH, Parsons R. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–1947. - PubMed
-
- Steck PA, Pershouse MA, Jasser SA, Yung WK, Lin H, Ligon AH, Langford LA, Baumgard ML, Hattier T, Davis T, Frye C, Hu R, Swedlund B, Teng DH, Tavtigian SV. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat. Genet. 1997;15:356–362. - PubMed
-
- Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J. Biol. Chem. 1998;273:13375–13378. - PubMed
-
- Huang H, Tindall DJ. Dynamic FoxO transcription factors. J. Cell Sci. 2007;120:2479–2487. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

