Update on morphea: part II. Outcome measures and treatment
- PMID: 21238824
- PMCID: PMC7382896
- DOI: 10.1016/j.jaad.2010.05.046
Update on morphea: part II. Outcome measures and treatment
Abstract
Morphea is a rare fibrosing disorder of the skin and underlying tissues. The underlying pathogenesis of morphea is not completely understood at this time, but ultimately results in an imbalance of collagen production and destruction. Evidence-based treatment options of morphea are limited secondary to the rarity of the disease, and the lack of universally used validated outcome measures. The most commonly used outcome measures are skin scores, computerized surface area measurement, durometer, cutometer, thermography, and ultrasound measurements. The Localized Scleroderma Cutaneous Assessment Tool is a promising recently validated skin scoring tool that allows differentiation between activity and damage, is sensitive to change, and requires no additional equipment. The most robust data in the treatment of morphea exists for methotrexate in combination with systemic steroids and ultraviolet A1.
Copyright © 2010 American Academy of Dermatology, Inc. Published by Mosby, Inc. All rights reserved.
Conflict of interest statement
The editors involved with this CME activity and all content validation/peer reviewers of this journal-based CME activity have reported no relevant financial relationships with commercial interest(s).
The authors of this journal-based CME activity have reported no relevant financial relationships with commercial interest(s).
The planners involved with this journal-based CME activity have reported no relevant financial relationships with commercial interest(s). The editorial and education staff involved with this journal-based CME activity have reported no relevant financial relationships with commercial interest(s).
In accordance with the ACCME Standards for Commercial Support of CME, the American Academy of Dermatology has implemented mechanisms, prior to the planning and implementation of this Journal-based CME activity, to identify and mitigate conflicts of interest for all individuals in a position to control the content of this Journal-based CME activity.
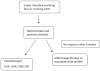
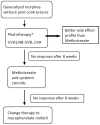
After completing this learning activity, participants should be able to recognize the need for a universally accepted validated outcome measure for the study of morphea therapies and compare the strengths and weaknesses of the currently available outcome measures, counsel their patients on the data available (and not available) for therapeutic options, and use the available treatment data and recommended therapeutic ladder when prescribing therapy.
Figures
References
-
- Hunzelmann N, Anders S, Fierlbeck G, Hein R, Herrmann K, Albrecht M, et al. Double-blind, placebo-controlled study of intralesional interferon gamma for the treatment of localized scleroderma. J Am Acad Dermatol. 1997;36:433–5. - PubMed
-
- Hulshof MM, Bouwes Bavinck JN, Bergman W, Masclee AA, Heickendorff L, Breedveld FC, et al. Double-blind, placebo-controlled study of oral calcitriol for the treatment of localized and systemic scleroderma. J Am Acad Dermatol. 2000;43:1017–23. - PubMed
-
- Kreuter A, Hyun J, Stucker M, Sommer A, Altmeyer P, Gambichler T. A randomized controlled study of low-dose UVA1, medium-dose UVA1, and narrowband UVB phototherapy in the treatment of localized scleroderma. J Am Acad Dermatol. 2006;54:440–7. - PubMed
-
- Kroft EB, Groeneveld TJ, Seyger MM, de Jong EM. Efficacy of topical tacrolimus 0.1% in active plaque morphea: randomized, double-blind, emollient-controlled pilot study. Am J Clin Dermatol. 2009;10:181–7. - PubMed
-
- Steen VD, Medsger TA, Jr, Rodnan GP. D-Penicillamine therapy in progressive systemic sclerosis (scleroderma): a retrospective analysis. Ann Intern Med. 1982;97:652–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical