Regulation of caspases in the nervous system implications for functions in health and disease
- PMID: 21238939
- PMCID: PMC9214546
- DOI: 10.1016/B978-0-12-385504-6.00007-5
Regulation of caspases in the nervous system implications for functions in health and disease
Abstract
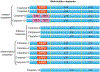
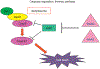
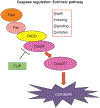
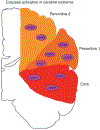
Caspases, initially identified as a family of proteases regulating cell death, have been found to have nonapoptotic functions as well. Some family members are critical for mediating programmed cell death in development. After development, caspases are downregulated in the nervous system, but continue to perform important nonapoptotic functions relevant for neurogenesis and synaptic plasticity. In neurodegenerative diseases, where aberrant neuronal death is an outstanding feature, there is an increase in caspase activity. The specific caspase death pathways leading to dysfunction and death have still not been fully clarified, despite the plethora of scientific literature addressing these issues. In this chapter, we will present the current knowledge of caspase activation and activity pathways, the current tools for examining caspases, and functions of caspases in the nervous system in health and in disease. Alzheimer's Disease, the most common neurodegenerative disorder, and cerebral ischemia, the most common cause of neurologic death, are used to illustrate our current understanding of death signaling in neurodegenerative diseases. A better understanding of how caspases function in health and disease would provide appropriate specific targets for the development of therapeutic interventions for these diseases. Life and death are exquisitely regulated at the cellular level from development through maturity. During development, neuronal death is the major factor shaping the nervous system. This death is mainly caspase-mediated apoptosis. Once the waves of developmental death have passed (death occurs at different times in different parts of the nervous system), there is downregulation of the death machinery, as the postmitotic neurons should live for the life of the organism. Aberrant neuronal death is a major part of neurodegenerative disorders, but there is still no clear understanding of the processes leading to the phenotypes of the various diseases. Even the type of death that occurs continues to be debated, whether it is apoptotic, necrotic, or autophagic, or some combination of these death mechanisms. Here, we will discuss the role that the caspases play in neuronal function, dysfunction, and death. First, we will discuss the regulation of caspase activation and activity. We will examine the current understanding of caspase function in developmental neuronal death and then illustrate the role of caspases in neuronal death in disease employing two diseases of neuronal loss, Alzheimer's Disease (AD), which is the most common chronic neurodegenerative disorder, and cerebral ischemia/stroke, the third most common cause of death in Western society, which is an acute neuronal disorder with chronic sequelae.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures


References
-
- Miura M, Zhu H, Rotello R, Hartwieg EA, Yuan J. Induction of apoptosis in fibroblasts by IL-1 beta-converting enzyme, a mammalian homolog of the C. elegans cell death gene ced-3. Cell 1993;75:653–60. - PubMed
-
- Thornberry NA, Bull HG, Calaycay JR, Chapman KT, Howard AD, Kostura MJ, et al. A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature 1992;356:768–74. - PubMed
-
- Yuan J, Shaham S, Ledoux S, Ellis HM, Horvitz HR. The C. elegans cell death gene ced-3 encodes a protein similar to mammalian interleukin-1 beta-converting enzyme. Cell 1993;75:641–52. - PubMed
-
- Eckhart L, Ban J, Fischer H, Tschachler E. Caspase-14: analysis of gene structure and mRNA expression during keratinocyte differentiation. Biochem Biophys Res Commun 2000;277:655–9. - PubMed
-
- Fischer H, Koenig U, Eckhart L, Tschachler E. Human caspase 12 has acquired deleterious mutations. Biochem Biophys Res Commun 2002;293:722–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

