Ronidazole pharmacokinetics after intravenous and oral immediate-release capsule administration in healthy cats
- PMID: 21239199
- PMCID: PMC10832812
- DOI: 10.1016/j.jfms.2010.12.001
Ronidazole pharmacokinetics after intravenous and oral immediate-release capsule administration in healthy cats
Abstract
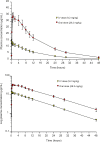
Ronidazole (RDZ) is an effective treatment for feline Tritrichomonas foetus infection, but has produced neurotoxicity in some cats. An understanding of the disposition of RDZ in cats is needed in order to make precise dosing recommendations. Single-dose pharmacokinetics of intravenous (IV) RDZ and immediate-release RDZ capsules were evaluated. A single dose of IV RDZ (mean 9.2mg/kg) and a 95mg immediate-release RDZ capsule (mean 28.2mg/kg) were administered to six healthy cats in a randomized crossover design. Plasma samples were collected for 48 h and assayed for RDZ using high pressure liquid chromatography (HPLC). Systemic absorption of oral RDZ was rapid and complete, with detection in the plasma of all cats by 10 min after dosing and a bioavailability of 99.64 (±16.54)%. The clearance of RDZ following IV administration was 0.82 (±0.07) ml/kg/min. The terminal half-life was 9.80 (±0.35) and 10.50 (±0.82) h after IV and oral administration, respectively, with drug detectable in all cats 48h after both administrations. The high oral bioavailability of RDZ and slow elimination may predispose cats to neurotoxicity with twice-daily administration. Less frequent administration should be considered for further study of effective treatment of T foetus-infected cats.
Copyright © 2011 ISFM and AAFP. Published by Elsevier Ltd. All rights reserved.
Figures

References
-
- Tolbert M.K., Gookin J. Tritrichomonas foetus: a new agent of feline diarrhea, Compend Contin Educ Vet 31, 2009, 374–381. - PubMed
-
- Gookin J.L., Copple C.N., Papich M.G., et al. Efficacy of ronidazole for treatment of feline Tritrichomonas foetus infection, J Vet Intern Med 20, 2006, 536–543. - PubMed
-
- Foster D.M., Gookin J.L., Poore M.F., Stebbins M.E., Levy M.G. Outcome of cats with diarrhea and Tritrichomonas foetus infection, J Am Vet Med Assoc 225, 2004, 888–892. - PubMed
-
- Gookin J.L., Stauffer S.H., Coccaro M.R., Poore M.F., Levy M.G., Papich M.G. Efficacy of tinidazole for treatment of cats experimentally infected with Tritrichomonas foetus, Am J Vet Res 68, 2007, 1085–1088. - PubMed
-
- Gookin J.L., Breitschwerdt E.B., Levy M.G., Gager R.B., Benrud J.G. Diarrhea associated with trichomonosis in cats, J Am Vet Med Assoc 215, 1999, 1450–1454. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

