Cortisol synthesis in epidermis is induced by IL-1 and tissue injury
- PMID: 21239489
- PMCID: PMC3060481
- DOI: 10.1074/jbc.M110.188268
Cortisol synthesis in epidermis is induced by IL-1 and tissue injury
Abstract
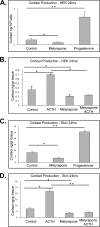
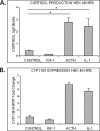
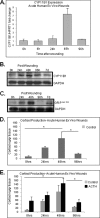
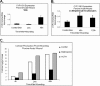
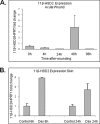
Glucocorticoids (GCs) are known inhibitors of wound healing. In this study we report the novel finding that both keratinocytes in vitro and epidermis in vivo synthesize cortisol and how this synthesis regulates wound healing. We show that epidermis expresses enzymes essential for cortisol synthesis, including steroid 11 β-hydroxylase (CYP11B1), and an enzyme that controls negative feedback mechanism, 11β-hydroxysteroid dehydrogenase 2 (11βHSD2). We also found that cortisol synthesis in keratinocytes and skin can be stimulated by ACTH and inhibited by metyrapone (CYP11B1 enzyme inhibitor). Interestingly, IL-1β, the first epidermal signal of tissue injury, induces the expression of CYP11B1 and increases cortisol production by keratinocytes. Additionally, we found induction of CYP11B1 increased production of cortisol and activation of GR pathway during wound healing ex vivo and in vivo using human and porcine wound models, respectively. Conversely, inhibition of cortisol synthesis during wound healing increases IL-1β production, suggesting that cortisol synthesis in epidermis may serve as a local negative feedback to proinflammatory cytokines. Local GCs synthesis, therefore, may provide control of the initial proinflammatory response, preventing excessive inflammation upon tissue injury. Inhibition of GC synthesis accelerated wound closure in vivo, providing the evidence that modulation of cortisol synthesis in epidermis may be an important regulatory mechanism during wound healing.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

