A role for autophagic protein beclin 1 early in lymphocyte development
- PMID: 21239722
- PMCID: PMC3070473
- DOI: 10.4049/jimmunol.1002223
A role for autophagic protein beclin 1 early in lymphocyte development
Abstract
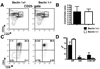
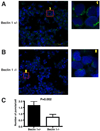
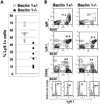
Autophagy is a highly regulated and evolutionarily conserved process of cellular self-digestion. Recent evidence suggests that this process plays an important role in regulating T cell homeostasis. In this study, we used Rag1(-/-) (recombination activating gene 1(-/-)) blastocyst complementation and in vitro embryonic stem cell differentiation to address the role of Beclin 1, one of the key autophagic proteins, in lymphocyte development. Beclin 1-deficient Rag1(-/-) chimeras displayed a dramatic reduction in thymic cellularity compared with control mice. Using embryonic stem cell differentiation in vitro, we found that the inability to maintain normal thymic cellularity is likely caused by impaired maintenance of thymocyte progenitors. Interestingly, despite drastically reduced thymocyte numbers, the peripheral T cell compartment of Beclin 1-deficient Rag1(-/-) chimeras is largely normal. Peripheral T cells displayed normal in vitro proliferation despite significantly reduced numbers of autophagosomes. In addition, these chimeras had greatly reduced numbers of early B cells in the bone marrow compared with controls. However, the peripheral B cell compartment was not dramatically impacted by Beclin 1 deficiency. Collectively, our results suggest that Beclin 1 is required for maintenance of undifferentiated/early lymphocyte progenitor populations. In contrast, Beclin 1 is largely dispensable for the initial generation and function of the peripheral T and B cell compartments. This indicates that normal lymphocyte development involves Beclin 1-dependent, early-stage and distinct, Beclin 1-independent, late-stage processes.
Figures




References
-
- Matsunaga K, Noda T, Yoshimori T. Binding Rubicon to cross the Rubicon. Autophagy. 2009;5:876–877. - PubMed
-
- Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, Packer M, Schneider MD, Levine B. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–939. - PubMed
-
- Furuya D, Tsuji N, Yagihashi A, Watanabe N. Beclin 1 augmented cis-diamminedichloroplatinum induced apoptosis via enhancing caspase-9 activity. Exp Cell Res. 2005;307:26–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

