Cdc42-dependent formation of the ZO-1/MRCKβ complex at the leading edge controls cell migration
- PMID: 21240187
- PMCID: PMC3041950
- DOI: 10.1038/emboj.2010.353
Cdc42-dependent formation of the ZO-1/MRCKβ complex at the leading edge controls cell migration
Abstract
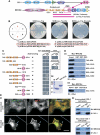
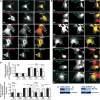
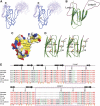
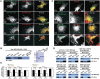
Zonula occludens (ZO)-1 is a multi-domain scaffold protein known to have critical roles in the establishment of cell-cell adhesions and the maintenance of stable tissue structures through the targeting, anchoring, and clustering of transmembrane adhesion molecules and cytoskeletal proteins. Here, we report that ZO-1 directly binds to MRCKβ, a Cdc42 effector kinase that modulates cell protrusion and migration, at the leading edge of migrating cells. Structural studies reveal that the binding of a β hairpin from GRINL1A converts ZO-1 ZU5 into a complete ZU5-fold. A similar interaction mode is likely to occur between ZO-1 ZU5 and MRCKβ. The interaction between ZO-1 and MRCKβ requires the kinase to be primed by Cdc42 due to the closed conformation of the kinase. Formation of the ZO-1/MRCKβ complex enriches the kinase at the lamellae of migrating cells. Disruption of the ZO-1/MRCKβ complex inhibits MRCKβ-mediated cell migration. These results demonstrate that ZO-1, a classical scaffold protein with accepted roles in maintaining cell-cell adhesions in stable tissues, also has an active role in cell migration during processes such as tissue development and remodelling.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


Similar articles
-
Association of ARVCF with zonula occludens (ZO)-1 and ZO-2: binding to PDZ-domain proteins and cell-cell adhesion regulate plasma membrane and nuclear localization of ARVCF.Mol Biol Cell. 2004 Dec;15(12):5503-15. doi: 10.1091/mbc.e04-04-0350. Epub 2004 Sep 29. Mol Biol Cell. 2004. PMID: 15456900 Free PMC article.
-
Structural changes in the carboxyl terminus of the gap junction protein connexin43 indicates signaling between binding domains for c-Src and zonula occludens-1.J Biol Chem. 2004 Dec 24;279(52):54695-701. doi: 10.1074/jbc.M409552200. Epub 2004 Oct 18. J Biol Chem. 2004. PMID: 15492000
-
Moxifloxacin induces random migration in human corneal fibroblasts via the protein kinase C epsilon/zonula occludens-1 signaling pathway.Eur J Pharmacol. 2021 Nov 5;910:174414. doi: 10.1016/j.ejphar.2021.174414. Epub 2021 Aug 20. Eur J Pharmacol. 2021. PMID: 34425101
-
Zonula occludens (ZO)-1 and ZO-2: membrane-associated guanylate kinase homologues (MAGuKs) of the tight junction.Biochem Soc Trans. 1995 Aug;23(3):470-5. doi: 10.1042/bst0230470. Biochem Soc Trans. 1995. PMID: 8566365 Review. No abstract available.
-
Myotonic dystrophy kinase-related Cdc42-binding kinases (MRCK), the ROCK-like effectors of Cdc42 and Rac1.Small GTPases. 2015;6(2):81-8. doi: 10.1080/21541248.2014.1000699. Epub 2015 Jun 19. Small GTPases. 2015. PMID: 26090570 Free PMC article. Review.
Cited by
-
LncRNA Bmp1 promotes the healing of intestinal mucosal lesions via the miR-128-3p/PHF6/PI3K/AKT pathway.Cell Death Dis. 2021 Jun 9;12(6):595. doi: 10.1038/s41419-021-03879-2. Cell Death Dis. 2021. PMID: 34108447 Free PMC article.
-
The Concise Guide to PHARMACOLOGY 2013/14: enzymes.Br J Pharmacol. 2013 Dec;170(8):1797-867. doi: 10.1111/bph.12451. Br J Pharmacol. 2013. PMID: 24528243 Free PMC article.
-
Epithelial polarization in the 3D matrix requires MST3 signaling to regulate ZO-1 position.PLoS One. 2023 May 8;18(5):e0285217. doi: 10.1371/journal.pone.0285217. eCollection 2023. PLoS One. 2023. PMID: 37155619 Free PMC article.
-
Cingulin binds to the ZU5 domain of scaffolding protein ZO-1 to promote its extended conformation, stabilization, and tight junction accumulation.J Biol Chem. 2022 Apr;298(4):101797. doi: 10.1016/j.jbc.2022.101797. Epub 2022 Mar 5. J Biol Chem. 2022. PMID: 35259394 Free PMC article.
-
The Cdc42 Effector Kinase PAK4 Localizes to Cell-Cell Junctions and Contributes to Establishing Cell Polarity.PLoS One. 2015 Jun 11;10(6):e0129634. doi: 10.1371/journal.pone.0129634. eCollection 2015. PLoS One. 2015. PMID: 26068882 Free PMC article.
References
-
- Ackerman SL, Kozak LP, Przyborski SA, Rund LA, Boyer BB, Knowles BB (1997) The mouse rostral cerebellar malformation gene encodes an UNC-5-like protein. Nature 386: 838–842 - PubMed
-
- Anderson JM, Fanning AS, Lapierre L, Van Itallie CM (1995) Zonula occludens (ZO)-1 and ZO-2: membrane-associated guanylate kinase homologues (MAGuKs) of the tight junction. Biochem Soc Trans 23: 470–475 - PubMed
-
- Anderson JM, Van Itallie CM, Fanning AS (2004) Setting up a selective barrier at the apical junction complex. Curr Opin Cell Biol 16: 140–145 - PubMed
-
- Balda MS, Matter K (1998) Tight junctions. J Cell Sci 111 (Part 5): 541–547 - PubMed
-
- Bax A, Grzesiek S (1993) Methodological advaces in protein NMR. Acc Chem Res 26: 131–138
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

