Time-lapse imaging of disease progression in deep brain areas using fluorescence microendoscopy
- PMID: 21240263
- PMCID: PMC3833825
- DOI: 10.1038/nm.2292
Time-lapse imaging of disease progression in deep brain areas using fluorescence microendoscopy
Abstract
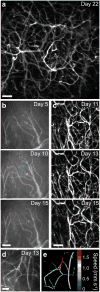
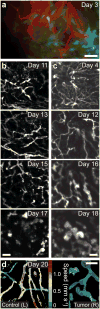
The combination of intravital microscopy and animal models of disease has propelled studies of disease mechanisms and treatments. However, many disorders afflict tissues inaccessible to light microscopy in live subjects. Here we introduce cellular-level time-lapse imaging deep within the live mammalian brain by one- and two-photon fluorescence microendoscopy over multiple weeks. Bilateral imaging sites allowed longitudinal comparisons within individual subjects, including of normal and diseased tissues. Using this approach, we tracked CA1 hippocampal pyramidal neuron dendrites in adult mice, revealing these dendrites' extreme stability and rare examples of their structural alterations. To illustrate disease studies, we tracked deep lying gliomas by observing tumor growth, visualizing three-dimensional vasculature structure and determining microcirculatory speeds. Average erythrocyte speeds in gliomas declined markedly as the disease advanced, notwithstanding significant increases in capillary diameters. Time-lapse microendoscopy will be applicable to studies of numerous disorders, including neurovascular, neurological, cancerous and trauma-induced conditions.
Figures



References
-
- Bullen A. Microscopic imaging techniques for drug discovery. Nat Rev Drug Discov. 2008;7:54–67. - PubMed
-
- Melder RJ, Salehi HA, Jain RK. Interaction of activated natural killer cells with normal and tumor vessels in cranial windows in mice. Microvasc Res. 1995;50:35–44. - PubMed
-
- Grutzendler J, Kasthuri N, Gan WB. Long-term dendritic spine stability in the adult cortex. Nature. 2002;420:812–816. - PubMed
-
- Trachtenberg JT, et al. Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature. 2002;420:788–794. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

