Efficient mucosal vaccination mediated by the neonatal Fc receptor
- PMID: 21240266
- PMCID: PMC3197702
- DOI: 10.1038/nbt.1742
Efficient mucosal vaccination mediated by the neonatal Fc receptor
Abstract
Almost all infectious diseases are initiated at mucosal surfaces, yet intramuscular or subcutaneous vaccination usually provides only minimal protection at sites of infection owing to suboptimal activation of the mucosal immune system. The neonatal Fc receptor (FcRn) mediates the transport of IgG across polarized epithelial cells lining mucosal surfaces. We mimicked this process by fusing a model antigen, herpes simplex virus type-2 (HSV-2) glycoprotein gD, to an IgG Fc fragment. Intranasal immunization, together with the adjuvant CpG, completely protected wild-type, but not FcRn knockout, mice after intravaginal challenge with virulent HSV-2 186. This immunization strategy induced efficient mucosal and systemic antibody, B- and T-cell immune responses, with stable protection for at least 6 months after vaccination in most of the immunized animals. The FcRn-IgG transcellular transport pathway may provide a general delivery route for subunit vaccines against many mucosal pathogens.
Figures
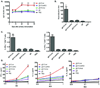
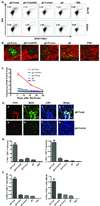
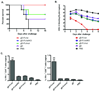
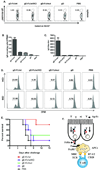
Comment in
-
A mucosal gateway for vaccines.Nat Biotechnol. 2011 Feb;29(2):136-8. doi: 10.1038/nbt.1766. Nat Biotechnol. 2011. PMID: 21301439 No abstract available.
References
-
- Neutra MR, Kozlowski PA. Mucosal vaccines: the promise and the challenge. Nat. Rev. Immunol. 2006;6:148–158. - PubMed
-
- Holmgren J, Czerkinsky C. Mucosal immunity and vaccines. Nat. Med. 2005;11(4 Suppl):S45–S53. - PubMed
-
- McGhee JR, et al. The mucosal immune system: from fundamental concepts to vaccine development. Vaccine. 1992;10:75–88. - PubMed
-
- Gallichan WS, Rosenthal KL. Long-term immunity and protection against herpes simplex virus type 2 in the murine female genital tract after mucosal but not systemic immunization. J. Infect. Dis. 1988;177:1155–1161. - PubMed
-
- Neutra MR, Mantis NJ, Kraehenbuhl J-P. Collaboration of epithelial cells with organized mucosal lymphoid tissues. Nat. Immunol. 2001;2:1004–1009. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

