A processed noncoding RNA regulates an altruistic bacterial antiviral system
- PMID: 21240270
- PMCID: PMC4612426
- DOI: 10.1038/nsmb.1981
A processed noncoding RNA regulates an altruistic bacterial antiviral system
Abstract
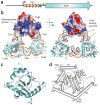
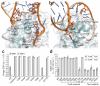
The ≥ 10³⁰ bacteriophages on Earth relentlessly drive adaptive coevolution, forcing the generation of protective mechanisms in their bacterial hosts. One such bacterial phage-resistance system, ToxIN, consists of a protein toxin (ToxN) that is inhibited in vivo by a specific RNA antitoxin (ToxI); however, the mechanisms for this toxicity and inhibition have not been defined. Here we present the crystal structure of the ToxN-ToxI complex from Pectobacterium atrosepticum, determined to 2.75-Å resolution. ToxI is a 36-nucleotide noncoding RNA pseudoknot, and three ToxI monomers bind to three ToxN monomers to generate a trimeric ToxN-ToxI complex. Assembly of this complex is mediated entirely through extensive RNA-protein interactions. Furthermore, a 2'-3' cyclic phosphate at the 3' end of ToxI, and catalytic residues, identify ToxN as an endoRNase that processes ToxI from a repetitive precursor but is regulated by its own catalytic product.
Figures


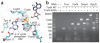
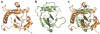
References
-
- Lima-Mendez G, Toussaint A, Leplae R. Analysis of the phage sequence space: the benefit of structured information. Virology. 2007;365:241–249. - PubMed
-
- Labrie SJ, Samson JE, Moineau S. Bacteriophage resistance mechanisms. Nat. Rev. Microbiol. 2010;8:317–327. - PubMed
-
- Chopin MC, Chopin A, Bidnenko E. Phage abortive infection in lactococci: variations on a theme. Curr. Opin. Microbiol. 2005;8:473–479. - PubMed
-
- Shub DA. Bacterial viruses. Bacterial altruism? Curr. Biol. 1994;4:555–556. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

