Structural basis of substrate discrimination and integrin binding by autotaxin
- PMID: 21240271
- PMCID: PMC3064516
- DOI: 10.1038/nsmb.1980
Structural basis of substrate discrimination and integrin binding by autotaxin
Abstract
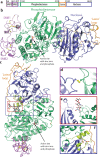
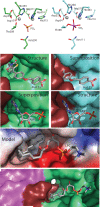
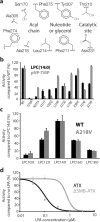
Autotaxin (ATX, also known as ectonucleotide pyrophosphatase/phosphodiesterase-2, ENPP2) is a secreted lysophospholipase D that generates the lipid mediator lysophosphatidic acid (LPA), a mitogen and chemoattractant for many cell types. ATX-LPA signaling is involved in various pathologies including tumor progression and inflammation. However, the molecular basis of substrate recognition and catalysis by ATX and the mechanism by which it interacts with target cells are unclear. Here, we present the crystal structure of ATX, alone and in complex with a small-molecule inhibitor. We have identified a hydrophobic lipid-binding pocket and mapped key residues for catalysis and selection between nucleotide and phospholipid substrates. We have shown that ATX interacts with cell-surface integrins through its N-terminal somatomedin B-like domains, using an atypical mechanism. Our results define determinants of substrate discrimination by the ENPP family, suggest how ATX promotes localized LPA signaling and suggest new approaches for targeting ATX with small-molecule therapeutic agents.
Figures


Comment in
-
Location, location, location: a crystal-clear view of autotaxin saturating LPA receptors.Nat Struct Mol Biol. 2011 Feb;18(2):117-8. doi: 10.1038/nsmb0211-117. Nat Struct Mol Biol. 2011. PMID: 21289650 Free PMC article.
References
-
- Stracke ML, et al. Identification, purification, and partial sequence analysis of autotaxin, a novel motility-stimulating protein. J Biol Chem. 1992;267:2524–2529. - PubMed
-
- Tokumura A, et al. Identification of human plasma lysophospholipase D, a lysophosphatidic acid-producing enzyme, as autotaxin, a multifunctional phosphodiesterase. J Biol Chem. 2002;277:39436–39442. - PubMed
-
- Noguchi K, Herr D, Mutoh T, Chun J. Lysophosphatidic acid (LPA) and its receptors. Curr Opin Pharmacol. 2009;9:15–23. - PubMed
-
- van Meeteren LA, Moolenaar WH. Regulation and biological activities of the autotaxin-LPA axis. Prog Lipid Res. 2007;46:145–160. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

