Restricted growth of U-type infectious haematopoietic necrosis virus (IHNV) in rainbow trout cells may be linked to casein kinase II activity
- PMID: 21241319
- PMCID: PMC7194290
- DOI: 10.1111/j.1365-2761.2010.01225.x
Restricted growth of U-type infectious haematopoietic necrosis virus (IHNV) in rainbow trout cells may be linked to casein kinase II activity
Abstract
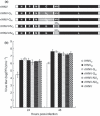
Previously, we demonstrated that a representative M genogroup type strain of infectious haematopoietic necrosis virus (IHNV) from rainbow trout grows well in rainbow trout-derived RTG-2 cells, but a U genogroup type strain from sockeye salmon has restricted growth, associated with reduced genome replication and mRNA transcription. Here, we analysed further the mechanisms for this growth restriction of U-type IHNV in RTG-2 cells, using strategies that assessed differences in viral genes, host immune regulation and phosphorylation. To determine whether the viral glycoprotein (G) or non-virion (NV) protein was responsible for the growth restriction, four recombinant IHNV viruses were generated in which the G gene of an infectious IHNV clone was replaced by the G gene of U- or M-type IHNV and the NV gene was replaced by NV of U- or M-type IHNV. There was no significant difference in the growth of these recombinants in RTG-2 cells, indicating that G and NV proteins are not major factors responsible for the differential growth of the U- and M-type strains. Poly I:C pretreatment of RTG-2 cells suppressed the growth of both U- and M-type IHNV, although the M virus continued to replicate at a reduced level. Both viruses induced type 1 interferon (IFN1) and the IFN1 stimulated gene Mx1, but the expression levels in M-infected cells were significantly higher than in U-infected cells and an inhibitor of the IFN1-inducible protein kinase PKR, 2-aminopurine (2-AP), did not affect the growth of U- or M-type IHNV in RTG-2 cells. These data did not indicate a role for the IFN1 system in the restricted growth of U-type IHNV in RTG-2 cells. Prediction of kinase-specific phosphorylation sites in the viral phosphoprotein (P) using the NetPhosK program revealed differences between U- and M-type P genes at five phosphorylation sites. Pretreatment of RTG-2 cells with a PKC inhibitor or a p38MAPK inhibitor did not affect the growth of the U- and M-type viruses. However, 100 μm of the casein kinase II (CKII) inhibitor, 5,6-dichloro-1-β-d-ribofuranosylbenzimidazole (DRB), reduced the titre of the U type 8.3-fold at 24 h post-infection. In contrast, 100 μm of the CKII inhibitor reduced the titre of the M type only 1.3-fold at 48 h post-infection. Our data suggest that the different growth of U- and M-type IHNV in RTG-2 cells may be linked to a differential requirement for cellular protein kinases such as CKII for their growth.
© 2011 Blackwell Publishing Ltd.
Figures


Similar articles
-
Differential growth of U and M type infectious haematopoietic necrosis virus in a rainbow trout-derived cell line, RTG-2.J Fish Dis. 2010 Jul;33(7):583-91. doi: 10.1111/j.1365-2761.2010.01153.x. Epub 2010 Mar 28. J Fish Dis. 2010. PMID: 20367739
-
Heterologous Exchanges of Glycoprotein and Non-Virion Protein in Novirhabdoviruses: Assessment of Virulence in Yellow Perch (Perca flavescens) and Rainbow Trout (Oncorhynchus mykiss).Viruses. 2024 Apr 22;16(4):652. doi: 10.3390/v16040652. Viruses. 2024. PMID: 38675990 Free PMC article.
-
Specificity of DNA vaccines against the U and M genogroups of infectious hematopoietic necrosis virus (IHNV) in rainbow trout (Oncorhynchus mykiss).Fish Shellfish Immunol. 2011 Jul;31(1):43-51. doi: 10.1016/j.fsi.2011.03.003. Epub 2011 Mar 6. Fish Shellfish Immunol. 2011. PMID: 21385613
-
Epidemiological characteristics of infectious hematopoietic necrosis virus (IHNV): a review.Vet Res. 2016 Jun 10;47(1):63. doi: 10.1186/s13567-016-0341-1. Vet Res. 2016. PMID: 27287024 Free PMC article. Review.
-
Immunity to fish rhabdoviruses.Viruses. 2012 Jan;4(1):140-66. doi: 10.3390/v4010140. Epub 2012 Jan 18. Viruses. 2012. PMID: 22355456 Free PMC article. Review.
Cited by
-
Impact of Vaccination and Pathogen Exposure Dosage on Shedding Kinetics of Infectious Hematopoietic Necrosis Virus (IHNV) in Rainbow Trout.J Aquat Anim Health. 2020 Sep;32(3):95-108. doi: 10.1002/aah.10108. Epub 2020 Aug 29. J Aquat Anim Health. 2020. PMID: 32443164 Free PMC article.
-
Comparative effects of Novirhabdovirus genes on modulating constitutive transcription and innate antiviral responses, in different teleost host cell types.Virol J. 2020 Jul 20;17(1):110. doi: 10.1186/s12985-020-01372-4. Virol J. 2020. PMID: 32690033 Free PMC article.
-
Naturally occurring substitution in one amino acid in VHSV phosphoprotein enhances viral virulence in flounder.PLoS Pathog. 2021 Jan 19;17(1):e1009213. doi: 10.1371/journal.ppat.1009213. eCollection 2021 Jan. PLoS Pathog. 2021. PMID: 33465148 Free PMC article.
-
Comparative transcriptome analysis of rainbow trout gonadal cells (RTG-2) infected with U and J genogroup infectious hematopoietic necrosis virus.Front Microbiol. 2023 Jan 17;13:1109606. doi: 10.3389/fmicb.2022.1109606. eCollection 2022. Front Microbiol. 2023. PMID: 36733771 Free PMC article.
-
Invitromatics, invitrome, and invitroomics: introduction of three new terms for in vitro biology and illustration of their use with the cell lines from rainbow trout.In Vitro Cell Dev Biol Anim. 2017 May;53(5):383-405. doi: 10.1007/s11626-017-0142-5. Epub 2017 Apr 3. In Vitro Cell Dev Biol Anim. 2017. PMID: 28374170 Review.
References
-
- Acosta F., Collet B., Lorenzen N. & Ellis A.E. (2006) Expression of the glycoprotein of viral haemorrhagic septicaemia virus (VHSV) on the surface of the fish cell line RTG‐P1 induces type 1 interferon expression in neighbouring cells. Fish and Shellfish Immunology 21, 272–278. - PubMed
-
- Ahmed M., McKenzie M.O., Puckett S., Hojnacki M., Poliquin L. & Lyles D.S. (2003) Ability of the matrix protein of vesicular stomatitis virus to suppress Beta interferon gene expression is genetically correlated with the inhibition of host RNA and protein synthesis. Journal of Virology 77, 4646–4657. - PMC - PubMed
-
- Balachandran S., Roberts P.C., Brown L.E., Truong H., Pattnaik A.K., Archer D.R. & Barber G.N. (2000) Essential role for the dsRNA‐dependent protein kinase PKR in innate immunity to viral infection. Immunity 13, 129–141. - PubMed
-
- Batts W.N. & Winton J.R. (1989) Enhanced detection of infectious hematopoietic necrosis virus and other fish viruses by pretreatment of cell monolayers with polyethylene glycol. Journal of Aquatic Animal Health 1, 284–290.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources

