A new topical formulation enhances relative diclofenac bioavailability in healthy male subjects
- PMID: 21241352
- PMCID: PMC3099372
- DOI: 10.1111/j.1365-2125.2011.03914.x
A new topical formulation enhances relative diclofenac bioavailability in healthy male subjects
Abstract
What is already known about this subject: • Therapy with topical non-steroidal anti-inflammatory drugs (NSAIDs) relies on the ability of the active drug to penetrate the skin in sufficiently high amounts to exert a clinical effect, which is linked to the specific galenic properties of the formulation.
What this study adds: • This phase 1 study characterizes the transdermal penetration and plasma exposure of different dose levels with galenic differences of a novel topical diclofenac formulation under development and indicates greater diclofenac penetration through the skin when compared with a commercially available formulation.
Aims: To evaluate the relative plasma and tissue availability of diclofenac after repeated topical administration of a novel diclofenac acid-based delivery system under development (DCF100C).
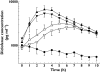
Methods: This was a single-centre, open-label, three-period, crossover clinical trial of five discrete diclofenac formulations. Test preparations comprised two concentrations (1.0% and 2.5%) of DCF100C, with and without menthol and eucalyptus oil (total daily doses of 5 mg and 12.5 mg). Voltaren Emulgel gel (1.0%) was the commercially available comparator (total daily dose of 40 mg). Topical application was performed onto the thigh of 20 male healthy subjects for 3 days. Applying a Youden square design, each drug was evaluated in 12 subjects, with each subject receiving three test preparations. Blood sampling and in vivo microdialysis in subcutaneous adipose and skeletal muscle tissues were performed for 10 h after additional final doses on the morning of day 4.
Results: All four DCF100C formulations demonstrated a three- to fivefold, dose-dependent increase in systemic diclofenac availability compared with Voltaren Emulgel and were approximately 30-40 times more effective at facilitating diclofenac penetration through the skin, taking different dose levels into account. Tissue concentrations were low and highly variable. The 2.5% DCF100C formulation without sensory excipients reached the highest tissue concentrations. AUC(0,10 h) was 2.71 times greater than for Voltaren Emulgel (90% CI 99.27, 737.46%). Mild erythema at the application site was the most frequent adverse event associated with DCF100C. There were no local symptoms after treatment with the reference formulation.
Conclusion: DCF100C formulations were safe and facilitated greater diclofenac penetration through the skin compared with the commercial comparator. DCF100C represents a promising alternative to oral and topical diclofenac treatments that warrants further development.
© 2011 The Authors. British Journal of Clinical Pharmacology © 2011 The British Pharmacological Society.
Figures
References
-
- European Medicines Agency. Available at http://www.emea.europa.eu/pdfs/human/press/pr/41313606.pdf (last accessed 13 July 2010)
-
- Zacher J, Altman R, Bellamy N, Brühlmann P, Da Silva J, Huskisson E, Taylor RS. Topical diclofenac and its role in pain and inflammation: an evidence-based review. Curr Med Res Opin. 2008;24:925–50. - PubMed
-
- Castelnuovo E, Cross P, Mt-Isa S, Spencer A, Underwood M. TOIB study team. Cost-effectiveness of advising the use of topical or oral ibuprofen for knee pain; the TOIB study [ISRCTN: 79353052] Rheumatology (Oxford) 2008;47:1077–81. - PubMed
-
- Barthel HR, Haselwood D, Longley S, 3rd, Gold MS, Altman RD. Randomized controlled trial of diclofenac sodium gel in knee osteoarthritis. Semin Arthritis Rheum. 2009;39:203–12. - PubMed
-
- Altman RD, Dreiser RL, Fisher CL, Chase WF, Dreher DS, Zacher J. Diclofenac sodium gel in patients with primary hand osteoarthritis: a randomized, double-blind, placebo-controlled trial. J Rheumatol. 2009;36:1991–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

