ERK1/2 deactivation enhances cytoplasmic Nur77 expression level and improves the apoptotic effect of fenretinide in human liver cancer cells
- PMID: 21241664
- PMCID: PMC3059345
- DOI: 10.1016/j.bcp.2011.01.005
ERK1/2 deactivation enhances cytoplasmic Nur77 expression level and improves the apoptotic effect of fenretinide in human liver cancer cells
Abstract
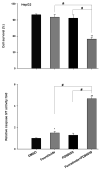
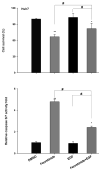
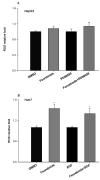
Fenretinide, a synthetic retinoid, is a promising anticancer agent based on many in vitro, animal, and chemoprevention clinical trial studies. However, cells such as HepG2 human liver cancer cells are resistant to the apoptotic effect of fenretinide. Previously, we have shown that fenretinide-induced apoptosis is Nur77 dependent, and the sensitivity of the cancer cells to fenretinide-induced apoptosis is positively associated with cytoplasmic enrichment of Nur77. The goal of current study was to identify means to modulate nuclear export of Nur77 in order to improve the efficacy of fenretinide. Fenretinide treatment deactivated ERK1/2 in Huh7 cells, but activated ERK1/2 in HepG2 cells, which was positively associated with the sensitivity of cells to the apoptotic effect of fenretinide. Neither fenretinide nor ERK1/2 inhibitor PD98059 alone could affect the survival of HepG2 cells, but the combination of both induced cell death and increased caspase 3/7 activity. In fenretinide sensitive Huh7 cells, activation of ERK1/2 by epidermal growth factor (EGF) prevented fenretinide-induced cell death and caspase 3/7 induction. In addition, modulation of ERK1/2 changed the intracellular localization of Nur77. Fenretinide/PD98059-induced cell death of HepG2 cell was positively associated with induction and cytoplasmic location as well as mitochondria enrichment of Nur77. The effect was specific for ERK1/2 because other mitogen activated protein kinases such as P38, Akt, and JNK did not have correlated changes in their phosphorylation levels. Taken together, the current study demonstrates that ERK1/2-modulated Nur77 intracellular location dictates the efficacy of fenretinide-induced apoptosis.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
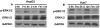




References
-
- Moon RC, Mehta RG. Chemoprevention of experimental carcinogenesis in animals. Prev Med. 1989;18:576–91. - PubMed
-
- Hail N, Jr, Kim HJ, Lotan R. Mechanisms of fenretinide-induced apoptosis. Apoptosis. 2006;11:1677–94. - PubMed
-
- Wu JM, DiPietrantonio AM, Hsieh TC. Mechanism of fenretinide (4-HPR)-induced cell death. Apoptosis. 2001;6:377–88. - PubMed
-
- Sogno I, Vene R, Ferrari N, De Censi A, Imperatori A, Noonan DM, et al. Angioprevention with fenretinide: targeting angiogenesis in prevention and therapeutic strategies. Crit Rev Oncol Hematol. 2010;75:2–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

