Hippocampal responsiveness to 17β-estradiol and equol after long-term ovariectomy: implication for a therapeutic window of opportunity
- PMID: 21241683
- PMCID: PMC3081673
- DOI: 10.1016/j.brainres.2011.01.029
Hippocampal responsiveness to 17β-estradiol and equol after long-term ovariectomy: implication for a therapeutic window of opportunity
Abstract
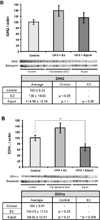
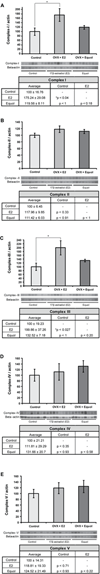
A 'critical window of opportunity' has been proposed for the efficacy of ovarian hormone intervention in peri- and post-menopausal women. We sought to address this hypothesis using a long-term ovariectomized non-human primate (NHP) model, the cynomolgus macaque (Macaca fascicularis). In these studies, we assessed the ability of 17β-estradiol and equol to regulate markers of hippocampal bioenergetic capacity. Results indicated that 17β-estradiol treatment significantly increased expression of mitochondrial respiratory chain proteins complex-I and -III in the hippocampus when compared to non-hormone-treated animals. Expression of the TCA cycle protein succinate dehydrogenase α was decreased in animals treated with equol compared to those treated with 17β-estradiol. There were no significant effects of either 17β-estradiol or equol treatment on glycolytic protein expression in the hippocampus, nor were there significant effects of treatment on expression levels of antioxidant enzymes. Similarly, 17β-estradiol and equol treatment had no effect on mitochondrial fission and fusion protein expression. In summary, findings indicate that while 17β-estradiol induced a significant increase in several proteins, the overall profile of bioenergetic system proteins was neutral to slightly positively responsive. The profile of responses with the ERβ-preferring molecule equol was consistent with overall nonresponsiveness. Collectively, the data indicate that long-term ovariectomy is associated with a decline in response to estrogens and estrogen-like compounds. By extension, the data are consistent with a primary tenet of the critical window hypothesis, i.e., that the brains of post-menopausal women ultimately lose their ability to respond positively to estrogenic stimulation.
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures




References
-
- Alzheimer's Association. Alzheimer's disease facts and figures. 2010 Mar - PubMed
-
- Appt SE, Clarkson TB, Register TC, Chen H. Dietary equol does not reproduce effects of dietary soy on cardiovascular disease risk variables; observations from postmenopausal monkeys. The North American Menopause Society; 16th Annual Meeting; September 28–October 1, 2005; San Diego. 2005.
-
- Barha CK, Galea LA. Motherhood alters the cellular response to estrogens in the hippocampus later in life. Neurobiol Aging. 2009 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

