Differentiation of human embryonic stem cells along a hepatic lineage
- PMID: 21241686
- PMCID: PMC3073319
- DOI: 10.1016/j.cbi.2011.01.009
Differentiation of human embryonic stem cells along a hepatic lineage
Abstract
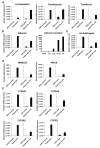
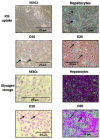
The limited availability of hepatic tissue suitable for the treatment of liver disease and drug discovery research advances the generation of hepatic-like cells from alternative sources as a valuable approach. In this investigation we exploited a unique hepatic differentiation approach to generate hepatocyte-like cells from human embryonic stem cells (hESCs). hESCs were cultured for 10-20 days on collagen substrate in highly defined and serum free hepatocyte media. The resulting cell populations exhibited hepatic cell-like morphology and were characterized with a variety of biological endpoint analyses. Real-time PCR analysis demonstrated that mRNA expression of the 'stemness' marker genes NANOG and alkaline phosphatase in the differentiated cells was significantly reduced, findings that were functionally validated using alkaline phosphatase activity detection measures. Immunofluorescence studies revealed attenuated levels of the 'stemness' markers OCT4, SOX2, SSEA-3, TRA-1-60, and TRA-1-81 in the hepatic-like cell population. The hepatic character of the cells was evaluated additionally by real-time PCR analyses that demonstrated increased mRNA expression of the hepatic transcription factors FOXA1, C/EBPα, and HNF1α, the nuclear receptors CAR, RXRα, PPARα, and HNF4α, the liver-generated plasma proteins α-fetoprotein, transthyretin, transferrin, and albumin, the protease inhibitor α-1-antitrypsin, metabolic enzymes HMGCS2, PEPCK, and biotransformation enzymes CYP3A7, CYP3A4, CYP3A5, and CYP2E1. Indocyanine green uptake albumin secretion and glycogen storage capacity further confirmed acquisition of hepatic function. These studies define an expeditious methodology that facilitates the differentiation of hESCs along a hepatic lineage and provide a framework for their subsequent use in pharmacological and toxicological research applications requiring a renewable supply of human hepatocytes.
2011 Elsevier Ireland Ltd. All rights reserved.
Figures





References
-
- Cao S, Esquivel CO, Keeffe EB. New approaches to supporting the failing liver. Annu Rev Med. 1998;49:85–94. - PubMed
-
- Malhi H, Gupta S. Hepatocyte transplantation: new horizons and challenges. J Hepatobiliary Pancreat Surg. 2001;8:40–50. - PubMed
-
- Schwartz RE, Linehan JL, Painschab MS, Hu WS, Verfaillie CM, Kaufman DS. Defined conditions for development of functional hepatic cells from human embryonic stem cells. Stem Cells Dev. 2005;14:643–55. - PubMed
-
- Lemaigre F, Zaret KS. Liver development update: new embryo models, cell lineage control, and morphogenesis. Curr Opin Genet Dev. 2004;14:582–90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

