Heat shock enhances CMV-IE promoter-driven metabotropic glutamate receptor expression and toxicity in transfected cells
- PMID: 21241715
- PMCID: PMC3380641
- DOI: 10.1016/j.neuropharm.2011.01.010
Heat shock enhances CMV-IE promoter-driven metabotropic glutamate receptor expression and toxicity in transfected cells
Abstract
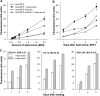
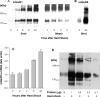
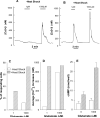
In CHO-K1 cells, heat shock strongly activated reporter-gene expression driven by the cytomegalovirus immediate-early (CMV-IE) promoter from adenoviral and plasmid vectors. Heat shock treatment (2h at 42.5 °C) significantly enhanced the promoter DNA-binding activity in nuclear extracts. In CHO cells expressing mGluR1a and mGluR5a receptors under the control of the CMV promoter, heat shock increased receptor protein expression, mRNA levels and receptor function estimated by measurement of PI hydrolysis, intracellular Ca²+ and cAMP. Hyperthermia increased average amplitudes of Ca²+ responses, the number of responding cells, and revealed the toxic properties of mGluR1a receptor. Heat shock also effectively increased the expression of EGFP. Hence, heat shock effects on mGluR expression and function in CHO cells may be attributed to the activation of the CMV promoter. Moreover, this effect was not limited to CHO cells as heat shock also increased EGFP expression in PC-12 and HEK293 cells. Heat shock treatment may be a useful tool to study the function of proteins expressed in heterologous systems under control of the CMV promoter. It may be especially valuable for increasing protein expression in transient transfections, for enhancing receptor expression in drug screening applications and to control the expression of proteins endowed with toxic properties. This article is part of a Special Issue entitled 'Trends in neuropharmacology: in memory of Erminio Costa'.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures

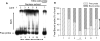

References
-
- Abe T, Sugihara H, Nawa H, Shigemoto R, Mizuno N, Nakanishi S. Molecular Characterization of a Novel Metabotropic Glutamate Receptor MGluR5 Coupled to Inositol Phosphate/Ca2+ Signal Transduction. J Biol Chem. 1992;267:13361–8. - PubMed
-
- Angulo A, Suto C, Heyman RA, Ghazal P. Characterization of the Sequences of the Human Cytomegalovirus Enhancer That Mediate Differential Regulation by Natural and Synthetic Retinoids. Mol Endocrinol. 1996;10:781–93. - PubMed
-
- Aramori I, Nakanishi S. Signal Transduction and Pharmacological Characteristics of a Metabotropic Glutamate Receptor, MGluR1, in Transfected CHO Cells. Neuron. 1992;8:757–65. - PubMed
-
- Boom R, Sol CJ, Minnaar RP, Geelen JL, Raap AK, van der Noordaa J. Induction of Gene Expression Under Human Cytomegalovirus Immediate Early Enhancer-Promoter Control by Inhibition of Protein Synthesis Is Cell Cycle-Dependent. J Gen Virol. 1988;69(Pt 6):1179–93. - PubMed
-
- Chan YJ, Chiou CJ, Huang Q, Hayward GS. Synergistic Interactions Between Overlapping Binding Sites for the Serum Response Factor and ELK-1 Proteins Mediate Both Basal Enhancement and Phorbol Ester Responsiveness of Primate Cytomegalovirus Major Immediate-Early Promoters in Monocyte and T-Lymphocyte Cell Types. J Virol. 1996;70:8590–605. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

