Positron emission tomography imaging of the stability of Cu-64 labeled dipalmitoyl and distearoyl lipids in liposomes
- PMID: 21241753
- PMCID: PMC3140766
- DOI: 10.1016/j.jconrel.2011.01.008
Positron emission tomography imaging of the stability of Cu-64 labeled dipalmitoyl and distearoyl lipids in liposomes
Abstract
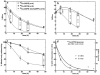
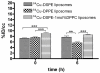
Changes in lipid acyl chain length can result in desorption of lipid from the liposomal anchorage and interaction with blood components. PET studies of the stability of such lipids have not been performed previously although such studies can map the pharmacokinetics of unstable lipids non-invasively in vivo. The purpose of this study was to characterize the in vivo clearance of (64)Cu-labeled distearoyl- and dipalmitoyl lipid included within long circulating liposomes. Distearoyl and dipalmitoyl maleimide lipids (1mol%) in liposomes were labeled with a (64)Cu-incorporated bifunctional chelator (TETA-PDP) after the activation of pyridine disulfide to thiol by TCEP. Long circulating liposomes containing HSPC:DSPE-PEG2k-OMe:cholesterol: x (55:5:39:1), where x was (64)Cu-DSPE or (64)Cu-DPPE, or HSPC:DSPE-PEG2k-OMe:cholesterol:(64)Cu-DSPE:DPPC (54:5:39:1:1) were evaluated in serum (in vitro) and via intravenous injection to FVB mice. The time-activity curves for the blood, liver, and kidney were measured from PET images and the biodistribution was performed at 48h. In vitro assays showed that (64)Cu-DPPE transferred from liposomes to serum with a 7.9h half-life but (64)Cu-DSPE remained associated with the liposomes. The half clearance of radioactivity from the blood pool was 18 and 5h for (64)Cu-DSPE- and (64)Cu-DPPE liposome-injected mice, respectively. The clearance of radioactivity from the liver and kidney was significantly greater following the injection of (64)Cu-DPPE-labeled liposomes than (64)Cu-DSPE-labeled liposomes at 6, 18 and 28h. Forty eight hours after injection, the whole body radioactivity was 57 and 17% ID/cc for (64)Cu-DSPE and (64)Cu-DPPE, respectively. These findings suggest that the acyl chain length of the radiolabel should be considered for liposomal PET studies and that PET is an effective tool for evaluating the stability of nanoformulations in vivo.
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures




References
-
- Medina OP, Zhu Y, Kairemo K. Targeted liposomal drug delivery in cancer. Curr. Pharm. Des. 2004;10:2981–2989. - PubMed
-
- Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discovery. 2005;4:145–160. - PubMed
-
- Abu Lila AS, Ishida T, Kiwada H. Recent advances in tumor vasculature targeting using liposomal drug delivery systems. Expert Opin. Drug Deliv. 2009;6:1297–1309. - PubMed
-
- Abu Lila AS, Ishida T, Kiwada H. Targeting anticancer drugs to tumor vasculature using cationic liposomes. Pharm. Res. 2010;27:1171–1183. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

