Nuclear PTEN regulates the APC-CDH1 tumor-suppressive complex in a phosphatase-independent manner
- PMID: 21241890
- PMCID: PMC3249980
- DOI: 10.1016/j.cell.2010.12.020
Nuclear PTEN regulates the APC-CDH1 tumor-suppressive complex in a phosphatase-independent manner
Abstract
PTEN is a frequently mutated tumor suppressor gene that opposes the PI3K/AKT pathway through dephosphorylation of phosphoinositide-3,4,5-triphosphate. Recently, nuclear compartmentalization of PTEN was found as a key component of its tumor-suppressive activity; however its nuclear function remains poorly defined. Here we show that nuclear PTEN interacts with APC/C, promotes APC/C association with CDH1, and thereby enhances the tumor-suppressive activity of the APC-CDH1 complex. We find that nuclear exclusion but not phosphatase inactivation of PTEN impairs APC-CDH1. This nuclear function of PTEN provides a straightforward mechanistic explanation for the fail-safe cellular senescence response elicited by acute PTEN loss and the tumor-suppressive activity of catalytically inactive PTEN. Importantly, we demonstrate that PTEN mutant and PTEN null states are not synonymous as they are differentially sensitive to pharmacological inhibition of APC-CDH1 targets such as PLK1 and Aurora kinases. This finding identifies a strategy for cancer patient stratification and, thus, optimization of targeted therapies. PAPERCLIP:
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures

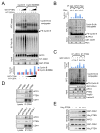
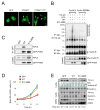
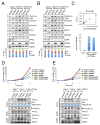

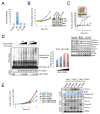

Comment in
-
Cancer biology: Role of nuclear PTEN revealed.Nat Rev Mol Cell Biol. 2011 Mar;12(3):134. doi: 10.1038/nrm3069. Epub 2011 Feb 16. Nat Rev Mol Cell Biol. 2011. PMID: 21326198 No abstract available.
-
Tumour suppressors: Role of nuclear PTEN revealed.Nat Rev Cancer. 2011 Mar;11(3):154. doi: 10.1038/nrc3028. Nat Rev Cancer. 2011. PMID: 21451549 No abstract available.
References
-
- Ali IU, Schriml LM, Dean M. Mutational spectra of PTEN/MMAC1 gene: a tumor suppressor with lipid phosphatase activity. J Natl Cancer Inst. 1999;91:1922–1932. - PubMed
-
- Blanco-Aparicio C, Renner O, Leal JF, Carnero A. PTEN, more than the AKT pathway. Carcinogenesis. 2007;28:1379–1386. - PubMed
-
- Cairns P, Okami K, Halachmi S, Halachmi N, Esteller M, Herman JG, Jen J, Isaacs WB, Bova GS, Sidransky D. Frequent inactivation of PTEN/MMAC1 in primary prostate cancer. Cancer Res. 1997;57:4997–5000. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

