RIM proteins tether Ca2+ channels to presynaptic active zones via a direct PDZ-domain interaction
- PMID: 21241895
- PMCID: PMC3063406
- DOI: 10.1016/j.cell.2010.12.029
RIM proteins tether Ca2+ channels to presynaptic active zones via a direct PDZ-domain interaction
Abstract
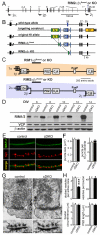
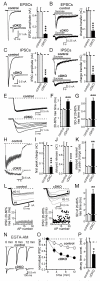
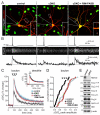
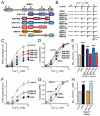
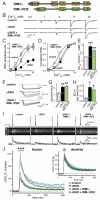
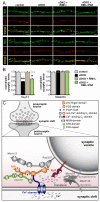
At a synapse, fast synchronous neurotransmitter release requires localization of Ca(2+) channels to presynaptic active zones. How Ca(2+) channels are recruited to active zones, however, remains unknown. Using unbiased yeast two-hybrid screens, we here identify a direct interaction of the central PDZ domain of the active-zone protein RIM with the C termini of presynaptic N- and P/Q-type Ca(2+) channels but not L-type Ca(2+) channels. To test the physiological significance of this interaction, we generated conditional knockout mice lacking all multidomain RIM isoforms. Deletion of RIM proteins ablated most neurotransmitter release by simultaneously impairing the priming of synaptic vesicles and by decreasing the presynaptic localization of Ca(2+) channels. Strikingly, rescue of the decreased Ca(2+)-channel localization required the RIM PDZ domain, whereas rescue of vesicle priming required the RIM N terminus. We propose that RIMs tether N- and P/Q-type Ca(2+) channels to presynaptic active zones via a direct PDZ-domain-mediated interaction, thereby enabling fast, synchronous triggering of neurotransmitter release at a synapse.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures


Comment in
-
The multiple faces of RIM.Neuron. 2011 Jan 27;69(2):185-7. doi: 10.1016/j.neuron.2011.01.010. Neuron. 2011. PMID: 21262457
References
-
- Betz A, Thakur P, Junge HJ, Ashery U, Rhee JS, Scheuss V, Rosenmund C, Rettig J, Brose N. Functional interaction of the active zone proteins Munc13-1 and RIM1 in synaptic vesicle priming. Neuron. 2001;30:183–196. - PubMed
-
- Castillo PE, Schoch S, Schmitz F, Sudhof TC, Malenka RC. RIM1alpha is required for presynaptic long-term potentiation. Nature. 2002;415:327–330. - PubMed
-
- Castillo PE, Weisskopf MG, Nicoll RA. The role of Ca2+ channels in hippocampal mossy fiber synaptic transmission and long-term potentiation. Neuron. 1994;12:261–269. - PubMed
-
- Catterall WA, Perez-Reyes E, Snutch TP, Striessnig J. International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol Rev. 2005;57:411–425. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

