Spatiotopic coding and remapping in humans
- PMID: 21242139
- PMCID: PMC3030833
- DOI: 10.1098/rstb.2010.0244
Spatiotopic coding and remapping in humans
Abstract
How our perceptual experience of the world remains stable and continuous in the face of continuous rapid eye movements still remains a mystery. This review discusses some recent progress towards understanding the neural and psychophysical processes that accompany these eye movements. We firstly report recent evidence from imaging studies in humans showing that many brain regions are tuned in spatiotopic coordinates, but only for items that are actively attended. We then describe a series of experiments measuring the spatial and temporal phenomena that occur around the time of saccades, and discuss how these could be related to visual stability. Finally, we introduce the concept of the spatio-temporal receptive field to describe the local spatiotopicity exhibited by many neurons when the eyes move.
Figures
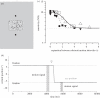
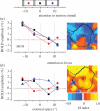
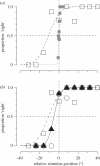
 .The index is constrained between −1 (full spatiotopicity) and +1 (full retinotopicity).
.The index is constrained between −1 (full spatiotopicity) and +1 (full retinotopicity).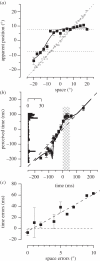
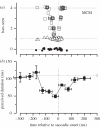

References
-
- Alhazen I. 1083. Book of optics. In The optics of Ibn al-Haytham (ed. Sabra A. I.). London, UK: Warburg Institute; (1989).
-
- Morgan M. J. 2003. The space between your ears: how the brain represents visual space. London, UK: Weidenfeld & Nicolson
-
- Galletti C., Battaglini P. P., Fattori P. 1993. Parietal neurons encoding spatial locations in craniotopic coordinates. Exptl. Brain Res. 96, 221–229 - PubMed
-
- Duhamel J., Bremmer F., BenHamed S., Graf W. 1997. Spatial invariance of visual receptive fields in parietal cortex neurons. Nature 389, 845–84810.1038/39865 (doi:10.1038/39865) - DOI - DOI - PubMed
-
- Trotter Y., Celebrini S. 1999. Gaze direction controls response gain in primary visual-cortex neurons. Nature 398, 239–24210.1038/18444 (doi:10.1038/18444) - DOI - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
