Remapping for visual stability
- PMID: 21242141
- PMCID: PMC3030834
- DOI: 10.1098/rstb.2010.0248
Remapping for visual stability
Abstract
Visual perception is based on both incoming sensory signals and information about ongoing actions. Recordings from single neurons have shown that corollary discharge signals can influence visual representations in parietal, frontal and extrastriate visual cortex, as well as the superior colliculus (SC). In each of these areas, visual representations are remapped in conjunction with eye movements. Remapping provides a mechanism for creating a stable, eye-centred map of salient locations. Temporal and spatial aspects of remapping are highly variable from cell to cell and area to area. Most neurons in the lateral intraparietal area remap stimulus traces, as do many neurons in closely allied areas such as the frontal eye fields the SC and extrastriate area V3A. Remapping is not purely a cortical phenomenon. Stimulus traces are remapped from one hemifield to the other even when direct cortico-cortical connections are removed. The neural circuitry that produces remapping is distinguished by significant plasticity, suggesting that updating of salient stimuli is fundamental for spatial stability and visuospatial behaviour. These findings provide new evidence that a unified and stable representation of visual space is constructed by redundant circuitry, comprising cortical and subcortical pathways, with a remarkable capacity for reorganization.
Figures


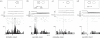


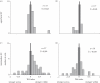
References
-
- Melcher D., Colby C. L. 2008. Trans-saccadic perception. Trends Cogn. Sci. 12, 466–47310.1016/j.tics.2008.09.003 (doi:10.1016/j.tics.2008.09.003) - DOI - DOI - PubMed
-
- Duhamel J. R., Colby C. L., Goldberg M. E. 1992. The updating of the representation of visual space in parietal cortex by intended eye movements. Science 255, 90–9210.1126/science.1553535 (doi:10.1126/science.1553535) - DOI - DOI - PubMed
-
- Nakamura K., Colby C. L. 2002. Updating of the visual representation in monkey striate and extrastriate cortex during saccades. Proc. Natl Acad. Sci. USA 99, 4026–403110.1073/pnas.052379899 (doi:10.1073/pnas.052379899) - DOI - DOI - PMC - PubMed
-
- Sommer M. A., Wurtz R. H. 2006. Influence of the thalamus on spatial visual processing in frontal cortex. Nature 444, 374–37710.1038/nature05279 (doi:10.1038/nature05279) - DOI - DOI - PubMed
-
- Kusunoki M., Goldberg M. E. 2003. The time course of perisaccadic receptive field shifts in the lateral intraparietal area of the monkey. J. Neurophysiol. 89, 1519–152710.1152/jn.00519.2002 (doi:10.1152/jn.00519.2002) - DOI - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

