L-Dopa activates histaminergic neurons
- PMID: 21242252
- PMCID: PMC3082096
- DOI: 10.1113/jphysiol.2010.203257
L-Dopa activates histaminergic neurons
Abstract
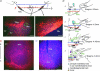
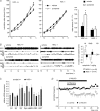
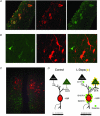
L-Dopa is the most effective treatment of early and advanced stages of Parkinson's disease (PD), but its chronic use leads to loss of efficiency and dyskinesia. This is delayed by lower dosage at early stages, made possible by additional treatment with histamine antagonists. We present here evidence that histaminergic tuberomamillary nucleus (TMN) neurons, involved in the control of wakefulness, are excited under L-Dopa (EC50 15 μM), express Dopa decarboxylase and show dopamine immunoreactivity. Dopaergic excitation was investigated with patch-clamp recordings from brain slices combined with single-cell RT-PCR analysis of dopamine receptor expression. In addition to the excitatory dopamine 1 (D1)-like receptors, TMN neurons express D2-like receptors, which are coupled through phospholipase C (PLC) to transient receptor potential canonical (TRPC) channels and the Na+/Ca2+ exchanger. D2 receptor activation enhances firing frequency, histamine release in freely moving rats (microdialysis) and wakefulness (EEG recordings). In histamine deficient mice the wake-promoting action of the D2 receptor agonist quinpirole (1 mg kg⁻¹, I.P.) is missing. Thus the histamine neurons can, subsequent to L-Dopa uptake, co-release dopamine and histamine from their widely projecting axons. Taking into consideration the high density of histaminergic fibres and the histamine H3 receptor heteromerization either with D1 or with D2 receptors in the striatum, this study predicts new avenues for PD therapy.
Figures




References
-
- Anaclet C, Parmentier R, Ouk K, Guidon G, Buda C, Sastre JP, Akaoka H, Sergeeva OA, Yanagisawa M, Ohtsu H, Franco P, Haas HL, Lin JS. Orexin/hypocretin and histamine: distinct roles in the control of wakefulness demonstrated using knock-out mouse models. J Neurosci. 2009;29:14423–14438. - PMC - PubMed
-
- Anichtchik OV, Rinne JO, Kalimo H, Panula P. An altered histaminergic innervation of the substantia nigra in Parkinson's disease. Exp Neurol. 2000;163:20–30. - PubMed
-
- Arbuthnott G, Wright AK. Some non-fluorescent connections of the nigro-neostriatal dopamine neurones. Brain Res Bull. 1982;9:367–368. - PubMed
-
- Arnulf I. Results of clinical trials of tiprolisant in narcolepsy and Parkinson's disease. Eur Neuropsychopharmacology. 2009;19:S204.
-
- Arnulf I, Leu-Semenescu S. Sleepiness in Parkinson's disease. Parkinsonism Relat Disord. 2009;15(Suppl 3):S101–S104. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

