The Drosophila blood brain barrier is maintained by GPCR-dependent dynamic actin structures
- PMID: 21242289
- PMCID: PMC3172179
- DOI: 10.1083/jcb.201007095
The Drosophila blood brain barrier is maintained by GPCR-dependent dynamic actin structures
Abstract
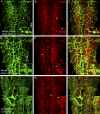
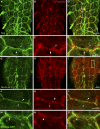
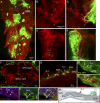
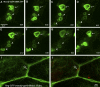
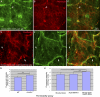
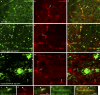
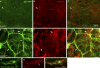
The blood brain barrier (BBB) is essential for insulation of the nervous system from the surrounding environment. In Drosophila melanogaster, the BBB is maintained by septate junctions formed between subperineurial glia (SPG) and requires the Moody/G protein-coupled receptor (GPCR) signaling pathway. In this study, we describe novel specialized actin-rich structures (ARSs) that dynamically form along the lateral borders of the SPG cells. ARS formation and association with nonmuscle myosin is regulated by Moody/GPCR signaling and requires myosin activation. Consistently, an overlap between ARS localization, elevated Ca(2+) levels, and myosin light chain phosphorylation is detected. Disruption of the ARS by inhibition of the actin regulator Arp2/3 complex leads to abrogation of the BBB. Our results suggest a mechanism by which the Drosophila BBB is maintained by Moody/GPCR-dependent formation of ARSs, which is supported by myosin activation. The localization of the ARSs close to the septate junctions enables efficient sealing of membrane gaps formed during nerve cord growth.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

