RAD21L, a novel cohesin subunit implicated in linking homologous chromosomes in mammalian meiosis
- PMID: 21242291
- PMCID: PMC3172173
- DOI: 10.1083/jcb.201008005
RAD21L, a novel cohesin subunit implicated in linking homologous chromosomes in mammalian meiosis
Abstract
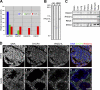
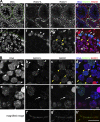
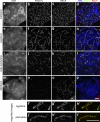
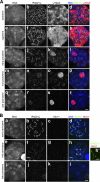
Cohesins are multi-subunit protein complexes that regulate sister chromatid cohesion during mitosis and meiosis. Here we identified a novel kleisin subunit of cohesins, RAD21L, which is conserved among vertebrates. In mice, RAD21L is expressed exclusively in early meiosis: it apparently replaces RAD21 in premeiotic S phase, becomes detectable on the axial elements in leptotene, and stays on the axial/lateral elements until mid pachytene. RAD21L then disappears, and is replaced with RAD21. This behavior of RAD21L is unique and distinct from that of REC8, another meiosis-specific kleisin subunit. Remarkably, the disappearance of RAD21L at mid pachytene correlates with the completion of DNA double-strand break repair and the formation of crossovers as judged by colabeling with molecular markers, γ-H2AX, MSH4, and MLH1. RAD21L associates with SMC3, STAG3, and either SMC1α or SMC1β. Our results suggest that cohesin complexes containing RAD21L may be involved in synapsis initiation and crossover recombination between homologous chromosomes.
Figures



References
-
- Anderson L.K., Royer S.M., Page S.L., McKim K.S., Lai A., Lilly M.A., Hawley R.S.. 2005. Juxtaposition of C(2)M and the transverse filament protein C(3)G within the central region of Drosophila synaptonemal complex. Proc. Natl. Acad. Sci. USA. 102:4482–4487. 10.1073/pnas.0500172102 - DOI - PMC - PubMed
-
- Barchi M., Mahadevaiah S., Di Giacomo M., Baudat F., de Rooij D.G., Burgoyne P.S., Jasin M., Keeney S.. 2005. Surveillance of different recombination defects in mouse spermatocytes yields distinct responses despite elimination at an identical developmental stage. Mol. Cell. Biol. 25:7203–7215. 10.1128/MCB.25.16.7203-7215.2005 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

