A single ubiquitin is sufficient for cargo protein entry into MVBs in the absence of ESCRT ubiquitination
- PMID: 21242292
- PMCID: PMC3172180
- DOI: 10.1083/jcb.201008121
A single ubiquitin is sufficient for cargo protein entry into MVBs in the absence of ESCRT ubiquitination
Abstract
ESCRTs (endosomal sorting complexes required for transport) bind and sequester ubiquitinated membrane proteins and usher them into multivesicular bodies (MVBs). As Ubiquitin (Ub)-binding proteins, ESCRTs themselves become ubiquitinated. However, it is unclear whether this regulates a critical aspect of their function or is a nonspecific consequence of their association with the Ub system. We investigated whether ubiquitination of the ESCRTs was required for their ability to sort cargo into the MVB lumen. Although we found that Rsp5 was the main Ub ligase responsible for ubiquitination of ESCRT-0, elimination of Rsp5 or elimination of the ubiquitinatable lysines within ESCRT-0 did not affect MVB sorting. Moreover, by fusing the catalytic domain of deubiquitinating peptidases onto ESCRTs, we could block ESCRT ubiquitination and the sorting of proteins that undergo Rsp5-dependent ubiquitination. Yet, proteins fused to a single Ub moiety were efficiently delivered to the MVB lumen, which strongly indicates that a single Ub is sufficient in sorting MVBs in the absence of ESCRT ubiquitination.
Figures

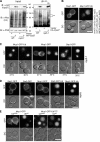
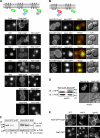
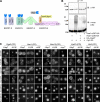

Comment in
-
Endocytosis: One ubiquitin does the trick.Nat Rev Mol Cell Biol. 2011 Mar;12(3):135. doi: 10.1038/nrm3063. Epub 2011 Feb 9. Nat Rev Mol Cell Biol. 2011. PMID: 21304550 No abstract available.
References
-
- Bilodeau P.S., Urbanowski J.L., Winistorfer S.C., Piper R.C. 2002. The Vps27p Hse1p complex binds ubiquitin and mediates endosomal protein sorting. Nat. Cell Biol. 4:534–539 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

