Intravital microscopy: new insights into metastasis of tumors
- PMID: 21242309
- PMCID: PMC3021994
- DOI: 10.1242/jcs.072728
Intravital microscopy: new insights into metastasis of tumors
Abstract
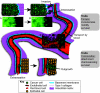
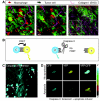
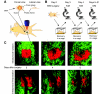
Metastasis, the process by which cells spread from the primary tumor to a distant site to form secondary tumors, is still not fully understood. Although histological techniques have provided important information, they give only a static image and thus compromise interpretation of this dynamic process. New advances in intravital microscopy (IVM), such as two-photon microscopy, imaging chambers, and multicolor and fluorescent resonance energy transfer imaging, have recently been used to visualize the behavior of single metastasizing cells at subcellular resolution over several days, yielding new and unexpected insights into this process. For example, IVM studies showed that tumor cells can switch between multiple invasion strategies in response to various densities of extracellular matrix. Moreover, other IVM studies showed that tumor cell migration and blood entry take place not only at the invasive front, but also within the tumor mass at tumor-associated vessels that lack an intact basement membrane. In this Commentary, we will give an overview of the recent advances in high-resolution IVM techniques and discuss some of the latest insights in the metastasis field obtained with IVM.
Figures


References
-
- Abdul-Karim M.-A., Al-Kofahi K., Brown E. B., Jain R. K., Roysam B. (2003). Automated tracing and change analysis of angiogenic vasculature from in vivo multiphoton confocal image time series. Microvasc. Res. 66, 113-125 - PubMed
-
- Alexander S., Koehl G., Hirschberg M., Geissler E., Friedl P. (2008). Dynamic imaging of cancer growth and invasion: a modified skin-fold chamber model. Histochem. Cell Biol. 130, 1147-1154 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

