A simian immunodeficiency virus macaque model of highly active antiretroviral treatment: viral latency in the periphery and the central nervous system
- PMID: 21242892
- PMCID: PMC3215305
- DOI: 10.1097/COH.0b013e3283412413
A simian immunodeficiency virus macaque model of highly active antiretroviral treatment: viral latency in the periphery and the central nervous system
Abstract
Purpose of review: Here, simian immunodeficiency virus (SIV) macaque models are examined for their strengths in identifying in-vivo sites of HIV latency and persistent virus replication during highly active antiretroviral treatment (HAART). The best characterized HIV reservoir in HAART-treated persons is resting CD4 T cells in blood, although residual virus also comes from other reservoirs. Nonhuman primate/SIV models of HAART have been developed to characterize potential HIV reservoirs, particularly the central nervous system (CNS) and stem cells in bone marrow, known and potential reservoirs of latent virus that are difficult to study in humans.
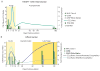
Recent findings: Few SIV macaque models of HAART have examined plasma and cerebrospinal fluid virus decay, the number of resting CD4 T cells harboring replication-competent latent SIV, HAART-treatment effect on the CNS, or residual viral replication or viral DNA levels in that tissue. Using a consistent, accelerated SIV macaque model, we characterized peripheral viral reservoirs, including those in the CNS, among HAART-treated macaques. The SIV model reproduces latency in memory CD4 T cells throughout the body and indicates that the CNS contains a stable SIV DNA reservoir.
Summary: An SIV macaque model of HAART recapitulating viral latency, particularly in the CNS, is required to study therapeutic approaches for a functional HIV cure.
Figures

References
-
- Dinoso JB, Rabi SA, Blankson JN, et al. A simian immunodeficiency virus-infected macaque model to study viral reservoirs that persist during highly active antiretroviral therapy. J Virol. 2009;83:9247–9257. This study establishes a SIV macaque model of HAART that reporduces many of the key features of HIV infection, including latent reservoirs. In the HAART-treated animals in this study, the frequency of circulating resting CD4+ T cells harboring replication-competent virus showed an initial decay similar to that observed for HAART-treated HIV-1-infected humans. - PMC - PubMed
-
- Sellier P, Mannioui A, Bourry O, et al. Antiretroviral treatment start-time during primary SIV(mac) infection in macaques exerts a different impact on early viral replication and dissemination. PLoS One. 2010;5:e10570. This study investigated timing of HAART initiation on viral replication in an SIV macaque model. - PMC - PubMed
-
- North TW, Higgins J, Deere JD, et al. Viral sanctuaries during highly active antiretroviral therapy in a nonhuman primate model for AIDS. J Virol. 2010;84:2913–2922. This study uses an SHIV macaque model to investigate latent viral reservoirs. Viral RNA and DNA were detected during HAART treatment in multiple tissue sources. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

