Transition metal catalyzed synthesis of aryl sulfides
- PMID: 21242940
- PMCID: PMC6259452
- DOI: 10.3390/molecules16010590
Transition metal catalyzed synthesis of aryl sulfides
Abstract
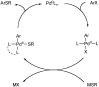
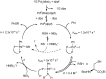
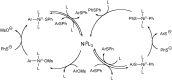
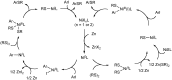
The presence of aryl sulfides in biologically active compounds has resulted in the development of new methods to form carbon-sulfur bonds. The synthesis of aryl sulfides via metal catalysis has significantly increased in recent years. Historically, thiolates and sulfides have been thought to plague catalyst activity in the presence of transition metals. Indeed, strong coordination of thiolates and thioethers to transition metals can often hinder catalytic activity; however, various catalysts are able to withstand catalyst deactivation and form aryl carbon-sulfur bonds in high-yielding transformations. This review discusses the metal-catalyzed arylation of thiols and the use of disulfides as metal-thiolate precursors for the formation of C-S bonds.
Figures





References
-
- Wang Y.G., Chackalamannil S., Hu Z.Y., Clader J.W., Greenlee W., Billard W., Binch H., Crosby G., Ruperto V., Duffy R.A., McQuade R., Lachowicz J.E. Design and synthesis of piperidinyl piperidine analogues as potent and selective M-2 muscarinic receptor antagonists. Biorg. Med. Chem. Lett. 2000;10:2247–2250. doi: 10.1016/S0960-894X(00)00457-1. - DOI - PubMed
-
- Liu G., Huth J.R., Olejniczak E.T., Mendoza R., DeVries P., Leitza S., Reilly E.B., Okasinski G.F., Fesik S.W., von Geldern T.W. Novel p-arylthio cinnamides as antagonists of leukocyte function-associated antigen-1/intracellular adhesion molecule-1 interaction. 2. Mechanism of inhibition and structure-based improvement of pharmaceutical properties. J. Med. Chem. 2001;44:1202–1210. doi: 10.1021/jm000503f. - DOI - PubMed
-
- Clader J.W., Billard W., Binch H., Chen L.Y., Crosby G., Duffy R.A., Ford J., Kozlowski J.A., Lachowicz J.E., Li S.J., Liu C., McCombie S.W., Vice S., Zhou G.W., Greenlee W.J. Muscarinic M2 antagonists: anthranilamide derivatives with exceptional selectivity and in vivo activity. Biorg. Med. Chem. 2004;12:319–326. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

