Monosaccharide-NAIM derivatives for D-, L-configurational analysis
- PMID: 21242944
- PMCID: PMC6259221
- DOI: 10.3390/molecules16010652
Monosaccharide-NAIM derivatives for D-, L-configurational analysis
Abstract
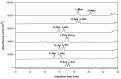
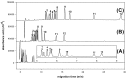
The D-, L-enantiomeric pairs of common monosaccharides (xylose, ribose, rhamnose, arabinose, fucose, glucose, mannose, galactose, N-acetylgalactosamine, glucuronic acid and galacturonic acid) were derivatized with 2,3-naphthalenediamine to form the corresponding D-, L-aldo-NAIM derivatives. A simple and facile capillary electrophoretic method was established for sugar composition analysis by simultaneously determining the migration times of these aldo-NAIMs using borate buffer at high pH (100 mM, pH 9.0). The methodology is also applicable to sialic acid (ketose monosaccharides). The quantitation level of the proposed method was in the 10~500 ppm range and the LOD was 1 ppm. The enantioseparation of D, L pairs of aldo-NAIMs were also achieved by using modified sulfated-α-cyclodextrin as the chiral selector in phosphate buffer (300 mM, pH 3.0). In addition, the combination by reductive amination of amino-aldo-NAIM agent and D-, L-enantiomeric pairs of monosaccharides formed a diastereomeric pair for saccharide configuration analysis. Aldo-NAIM derivatives are thus shown to be rapid and efficient agents for analyzing saccharide compositions and configurations with good linearity and short analysis times via capillary electrophoresis.
Figures






References
-
- Price N.P.J., Bowman M.J., Gall S.L., Berhow M.A., Kendra D.F., Lerouge P. Functionalized C-glycoside ketohydrazones: carbohydrate derivatives that retain the ring integrity of the terminal reducing sugar. Anal. Chem. 2010;82:2893–2899. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

