Plant-made vaccines in support of the Millennium Development Goals
- PMID: 21243362
- PMCID: PMC3075396
- DOI: 10.1007/s00299-010-0995-5
Plant-made vaccines in support of the Millennium Development Goals
Abstract
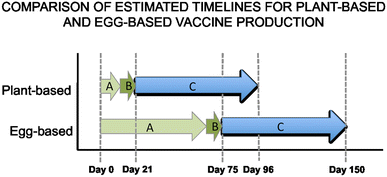
Vaccines are one of the most successful public health achievements of the last century. Systematic immunisation programs have reduced the burden of infectious diseases on a global scale. However, there are limitations to the current technology, which often requires costly infrastructure and long lead times for production. Furthermore, the requirement to keep vaccines within the cold-chain throughout manufacture, transport and storage is often impractical and prohibitively expensive in developing countries-the very regions where vaccines are most needed. In contrast, plant-made vaccines (PMVs) can be produced at a lower cost using basic greenhouse agricultural methods, and do not need to be kept within such narrow temperature ranges. This increases the feasibility of developing countries producing vaccines locally at a small-scale to target the specific needs of the region. Additionally, the ability of plant-production technologies to rapidly produce large quantities of strain-specific vaccine demonstrates their potential use in combating pandemics. PMVs are a proven technology that has the potential to play an important role in increasing global health, both in the context of the 2015 Millennium Development Goals and beyond.
Figures

References
-
- Atkinson WL, Pickering LK, Schwartz B, Weniger BG, Iskander JK, Watson JC. General recommendations on immunization. Recommendations of the Advisory Committee on Immunization Practices (ACIP) and the American Academy of Family Physicians (AAFP) MMWR Recomm Rep. 2002;51:1–36. - PubMed
-
- Barr IG, McCauley J, Cox N, Daniels R, Engelhardt OG, Fukuda K, Grohmann G, Hay A, Kelso A, Klimov A, Odagiri T, Smith D, Russell C, Tashiro M, Webby R, Wood J, Ye Z, Zhang W, Writing Committee of the WHO Consultation on Northern Hemisphere Influenza Vaccine Composition for 2009–2010 Epidemiological, antigenic and genetic characteristics of seasonal influenza A (H1N1) and B influenza viruses: basis for the WHO recommendation on the composition of influenza vaccines for use in the 2009–2010 Northern Hemisphere season. Vaccine. 2010;5:1156–1167. doi: 10.1016/j.vaccine.2009.11.043. - DOI - PubMed
-
- Bendandi M, Marillonnet S, Kandzia R, Thieme F, Nickstadt A, Herz S, Fröde R, Inogés S, Lòpez-Dìaz de Cerio A, Soria E, Villanueva H, Vancanneyt G, McCormick A, Tusé D, Lenz J, Butler-Ransohoff J-E, Klimyuk V, Gleba Y. Rapid, high-yield production in plants of individualized idiotype vaccines for non-Hodgkin’s lymphoma. Ann Oncol. 2010;21:2420–2427. doi: 10.1093/annonc/mdq256. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

