KAT(ching) metabolism by the tail: insight into the links between lysine acetyltransferases and metabolism
- PMID: 21243716
- PMCID: PMC3327878
- DOI: 10.1002/cbic.201000438
KAT(ching) metabolism by the tail: insight into the links between lysine acetyltransferases and metabolism
Abstract
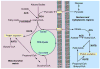
Post-translational modifications of histones elicit structural and functional changes within chromatin that regulate various epigenetic processes. Epigenetic mechanisms rely on enzymes whose activities are driven by coenzymes and metabolites from intermediary metabolism. Lysine acetyltransferases (KATs) catalyze the transfer of acetyl groups from acetyl-CoA to epsilon amino groups. Utilization of this critical metabolite suggests these enzymes are modulated by the metabolic status of the cell. This review highlights studies linking KATs to metabolism. We cover newly identified acyl modifications (propionylation and butyrylation), discuss the control of KAT activity by cellular acetyl-CoA levels, and provide insights into how acetylation regulates metabolic proteins. We conclude with a discussion of the current approaches to identifying novel KATs and their metabolic substrates.
Copyright © 2011 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures

References
-
- Kornberg RD, Lorch Y. Cell. 1999;98:285. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

