Osteoblast response to titanium surfaces functionalized with extracellular matrix peptide biomimetics
- PMID: 21244501
- PMCID: PMC4287399
- DOI: 10.1111/j.1600-0501.2010.02074.x
Osteoblast response to titanium surfaces functionalized with extracellular matrix peptide biomimetics
Abstract
Objective: Functionalizing surfaces with specific peptides may aid osteointegration of orthopedic implants by favoring attachment of osteoprogenitor cells and promoting osteoblastic differentiation. This study addressed the hypothesis that implant surfaces functionalized with peptides targeting multiple ligands will enhance osteoblast attachment and/or differentiation. To test this hypothesis, we used titanium (Ti) surfaces coated with poly-l-lysine-grafted polyethylene glycol (PLL-g-PEG) and functionalized with two peptides found in extracellular matrix proteins, arginine-glycine-aspartic acid (RGD) and lysine-arginine-serine-arginine (KRSR), which have been shown to increase osteoblast attachment. KSSR, which does not promote osteoblast attachment, was used as a control.
Materials and methods: Sandblasted acid-etched titanium surfaces were coated with PLL-g-PEG functionalized with varying combinations of RGD and KRSR, as well as KSSR. Effects of these surfaces on osteoblasts were assessed by measuring cell number, alkaline phosphatase-specific activity, and levels of osteocalcin, transforming growth factor beta-1 (TGF-β1), and PGE(2).
Results: RGD increased cell number, but decreased markers for osteoblast differentiation. KRSR alone had no effect on cell number, but decreased levels of TGF-β1 and PGE(2). KRSR and RGD/KRSR coatings inhibited osteoblast differentiation vs. PLL-g-PEG. KSSR decreased cell number and increased osteoblast differentiation, indicated by increased levels of osteocalcin and PGE(2).
Conclusions: The RGD and KRSR functionalized surfaces supported attachment but did not enhance osteoblast differentiation, whereas KSSR increased differentiation. RGD decreased this effect, suggesting that multifunctional peptide surfaces can be designed that improve peri-implant healing by optimizing attachment and proliferation as well as differentiation of osteoblasts, but peptide combination, dose and presentation are critical variables.
© 2011 John Wiley & Sons A/S.
Figures
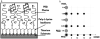
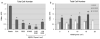

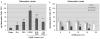


References
-
- Barber TA, Ho JE, De Ranieri A, Virdi AS, Sumner DR, Healy KE. Peri-implant bone formation and implant integration strength of peptide-modified p(aam-co-eg/aac) interpenetrating polymer network-coated titanium implants. Journal of Biomedical Materials Research A. 2007;80:306–320. - PubMed
-
- Boyan BD, Lohmann CH, Dean DD, Sylvia VL, Cochran DL, Schwartz Z. Mechanisms involved in osteoblast response to implant surface morphology. Annual Review of Materials Research. 2001;31:357–371.
-
- Boyan BD, Schwartz Z, Lohmann CH, Sylvia VL, Cochran DL, Dean DD, Puzas JE. Pretreatment of bone with osteoclasts affects phenotypic expression of osteoblastlike cells. Journal of Orthopaedic Research. 2003;21:638–647. - PubMed
-
- Buser D, Broggini N, Wieland M, Schenk RK, Denzer AJ, Cochran DL, Hoffmann B, Lussi A, Steinemann SG. Enhanced bone apposition to a chemically modified sla titaniumsurface. Journal of Dental Research. 2004;83:529–533. - PubMed
-
- Cardin AD, Weintraub HJR. Molecular modeling of protein-glycosaminoglycan interactions. Arteriosclerosis. 1989;9:21–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

