Calcium dynamics in the ventricular myocytes of SERCA2 knockout mice: A modeling study
- PMID: 21244828
- PMCID: PMC3021663
- DOI: 10.1016/j.bpj.2010.11.048
Calcium dynamics in the ventricular myocytes of SERCA2 knockout mice: A modeling study
Abstract
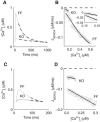
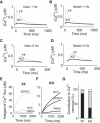
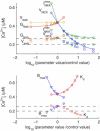
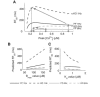
We describe a simulation study of Ca²(+) dynamics in mice with cardiomyocyte-specific conditional excision of the sarco(endo)plasmic reticulum calcium ATPase (SERCA) gene, using an experimental data-driven biophysically-based modeling framework. Previously, we reported a moderately impaired heart function measured in mice at 4 weeks after SERCA2 gene deletion (knockout (KO)), along with a >95% reduction in the level of SERCA2 protein. We also reported enhanced Ca²(+) flux through the L-type Ca²(+) channels and the Na(+)/Ca²(+) exchanger in ventricular myocytes isolated from these mice, compared to the control Serca2(flox/flox) mice (flox-flox (FF)). In the current study, a mathematical model-based analysis was applied to enable further quantitative investigation into changes in the Ca²(+) handling mechanisms in these KO cardiomyocytes. Model parameterization based on a wide range of experimental measurements showed a 67% reduction in SERCA activity and an over threefold increase in the activity of the Na(+)/Ca²(+) exchanger. The FF and KO models were then validated against experimentally measured [Ca²(+)](i) transients and experimentally estimated sarco(endo)plasmic reticulum (SR) function. Simulation results were in quantitative agreement with experimental measurements, confirming that sustained [Ca²(+)](i) transients could be maintained in the KO cardiomyocytes despite severely impaired SERCA function. In silico analysis shows that diastolic [Ca²(+)](i) rises sharply with progressive reductions in SERCA activity at physiologically relevant pacing frequencies. Furthermore, an analysis of the roles of the compensatory mechanisms revealed that the major combined effect of the compensatory mechanisms is to lower diastolic [Ca²(+)](i). Finally, by using a comprehensive sensitivity analysis of the role of all cellular calcium handling mechanisms, we show that the combination of upregulation of the Na(+)/Ca²(+) exchanger and increased L-type Ca²(+) current is the most effective means to maintain diastolic and systolic calcium levels after loss of SERCA function.
Copyright © 2011 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Feldman A.M., Weinberg E.O., Lorell B.H. Selective changes in cardiac gene expression during compensated hypertrophy and the transition to cardiac decompensation in rats with chronic aortic banding. Circ. Res. 1993;73:184–192. - PubMed
-
- Qi M., Shannon T.R., Samarel A.M. Downregulation of sarcoplasmic reticulum Ca(2+)-ATPase during progression of left ventricular hypertrophy. Am. J. Physiol. Heart Circ. Physiol. 1997;272:H2416–H2424. - PubMed
-
- Hasenfuss G., Reinecke H., Drexler H. Relation between myocardial function and expression of sarcoplasmic reticulum Ca(2+)-ATPase in failing and nonfailing human myocardium. Circ. Res. 1994;75:434–442. - PubMed
-
- Limas C.J., Olivari M.-T., Simon A. Calcium uptake by cardiac sarcoplasmic reticulum in human dilated cardiomyopathy. Cardiovasc. Res. 1987;21:601–605. - PubMed
-
- Pieske B., Maier L.S., Hasenfuss G. Ca2+ handling and sarcoplasmic reticulum Ca2+ content in isolated failing and nonfailing human myocardium. Circ. Res. 1999;85:38–46. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

